Assessing the Efficacy of First-Aid Measures in Physalia sp. Envenomation, Using Solution- and Blood Agarose-Based Models
- PMID: 28445412
- PMCID: PMC5450697
- DOI: 10.3390/toxins9050149
Assessing the Efficacy of First-Aid Measures in Physalia sp. Envenomation, Using Solution- and Blood Agarose-Based Models
Abstract
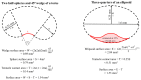
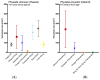
Stings from the hydrozoan species in the genus Physalia cause intense, immediate skin pain and elicit serious systemic effects. There has been much scientific debate about the most appropriate first aid for these stings, particularly with regard to whether vinegar use is appropriate (most current recommendations recommend against vinegar). We found that only a small percentage (≤1.0%) of tentacle cnidae discharge during a sting event using an ex vivo tissue model which elicits spontaneous stinging from live cnidarian tentacles. We then tested a variety of rinse solutions on both Atlantic and Pacific Physalia species to determine if they elicit cnidae discharge, further investigating any that did not cause immediate significant discharge to determine if they are able to inhibit cnidae discharge in response to chemical and physical stimuli. We found commercially available vinegars, as well as the recently developed Sting No More® Spray, were the most effective rinse solutions, as they irreversibly inhibited cnidae discharge. However, even slight dilution of vinegar reduced its protective effects. Alcohols and folk remedies, such as urine, baking soda and shaving cream, caused varying amounts of immediate cnidae discharge and failed to inhibit further discharge, and thus likely worsen stings.
Keywords: Portuguese man o’ war; bluebottle; cnidaria; first aid; hydrozoa; jellyfish; marine envenomation; sting.
Conflict of interest statement
A.A.Y. declares a potential conflict of interest as the inventor for USPTO applications PCT/US2012/000095 and PCT/US2015/037974. A.A.Y. is also the founder and principal of Alatalab Solutions, LLC, manufacturer of Sting No More® products. Management of this disclosed potential conflict of interest was achieved under an approved University of Hawaii Conflict of Interest (COI) plan. All aspects of the COI plan were followed while conducting this research study and in the independent analysis of data. C.L.W., J.L.H. and T.K.D. declare no conflicts of interest.
Figures



Similar articles
-
Experimental Assays to Assess the Efficacy of Vinegar and Other Topical First-Aid Approaches on Cubozoan (Alatina alata) Tentacle Firing and Venom Toxicity.Toxins (Basel). 2016 Jan 11;8(1):19. doi: 10.3390/toxins8010019. Toxins (Basel). 2016. PMID: 26761033 Free PMC article.
-
Cubozoan Sting-Site Seawater Rinse, Scraping, and Ice Can Increase Venom Load: Upending Current First Aid Recommendations.Toxins (Basel). 2017 Mar 15;9(3):105. doi: 10.3390/toxins9030105. Toxins (Basel). 2017. PMID: 28294982 Free PMC article.
-
First aid treatment of jellyfish stings in Australia. Response to a newly differentiated species.Med J Aust. 1993 Apr 5;158(7):498-501. doi: 10.5694/j.1326-5377.1993.tb137588.x. Med J Aust. 1993. PMID: 8469205
-
Toxic jellyfish in Thailand.Int Marit Health. 2019;70(1):22-26. doi: 10.5603/IMH.2019.0004. Int Marit Health. 2019. PMID: 30931514 Review.
-
Aquatic antagonists: Portuguese man-of-war (Physalia physalis).Cutis. 2007 Sep;80(3):186-8. Cutis. 2007. PMID: 17956005 Review. No abstract available.
Cited by
-
Effect of Rinse Solutions on Rhizostoma pulmo (Cnidaria: Scyphozoa) Stings and the Ineffective Role of Vinegar in Scyphozoan Jellyfish Species.Int J Environ Res Public Health. 2023 Jan 28;20(3):2344. doi: 10.3390/ijerph20032344. Int J Environ Res Public Health. 2023. PMID: 36767709 Free PMC article.
-
Field Experiment Effect on Citrus Spider Mite Panonychus citri of Venom from Jellyfish Nemopilema nomurai: The Potential Use of Jellyfish in Agriculture.Toxins (Basel). 2021 Jun 10;13(6):411. doi: 10.3390/toxins13060411. Toxins (Basel). 2021. PMID: 34200597 Free PMC article.
-
A systematic review of reports on aquatic envenomation: are there global hot spots and vulnerable populations?J Venom Anim Toxins Incl Trop Dis. 2024 Dec 20;30:e20240032. doi: 10.1590/1678-9199-JVATITD-2024-0032. eCollection 2024. J Venom Anim Toxins Incl Trop Dis. 2024. PMID: 39810839 Free PMC article. Review.
-
Inhibition of Nematocyst Discharge from Pelagia noctiluca (Cnidaria: Scyphozoa)-Prevention Measures against Jellyfish Stings.Mar Drugs. 2022 Sep 8;20(9):571. doi: 10.3390/md20090571. Mar Drugs. 2022. PMID: 36135760 Free PMC article.
-
Marine sentinels using eDNA to track Physalia sp. in the Gulf of Thailand.PLoS One. 2025 Jun 24;20(6):e0326215. doi: 10.1371/journal.pone.0326215. eCollection 2025. PLoS One. 2025. PMID: 40554571 Free PMC article.
References
-
- Halstead B.W. Poisonous and Venomous Marine Animals of the World. 2nd ed. Darwin Press; Princeton, NJ, USA: 1988. Coelenterates; pp. 99–186.
-
- Williamson J.A., Fenner P.J., Burnett J.W., Rifkin J.F. Venomous & Poisonous Marine Animals: A Medical and Biological Handbook. Surf Life Saving Australia and University of New South Wales Press; Sydney, Australia: 1996. pp. 120–311.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

