Delayed histochemical alterations within the neurovascular unit due to transient focal cerebral ischemia and experimental treatment with neurotrophic factors
- PMID: 28445478
- PMCID: PMC5405989
- DOI: 10.1371/journal.pone.0174996
Delayed histochemical alterations within the neurovascular unit due to transient focal cerebral ischemia and experimental treatment with neurotrophic factors
Abstract
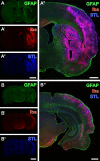
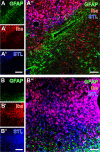
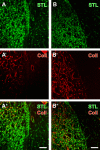
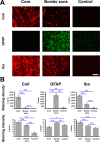
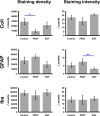
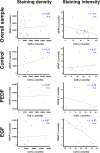
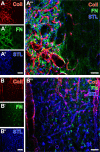
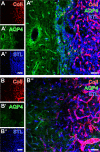
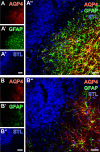
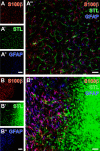
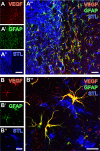
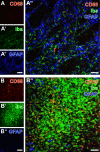
Current stroke therapy is focused on recanalizing strategies, but neuroprotective co-treatments are still lacking. Modern concepts of the ischemia-affected neurovascular unit (NVU) and surrounding penumbra emphasize the complexity during the transition from initial damaging to regenerative processes. While early treatment with neurotrophic factors was shown to result in lesion size reduction and blood-brain barrier (BBB) stabilization, cellular consequences from these treatments are poorly understood. This study explored delayed cellular responses not only to ischemic stroke, but also to an early treatment with neurotrophic factors. Rats underwent 60 minutes of focal cerebral ischemia. Fluorescence labeling was applied to sections from brains perfused 7 days after ischemia. Analyses focused on NVU constituents including the vasculature, astrocytes and microglia in the ischemic striatum, the border zone and the contralateral hemisphere. In addition to histochemical signs of BBB breakdown, a strong up-regulation of collagen IV and microglia activation occurred within the ischemic core with simultaneous degradation of astrocytes and their endfeet. Activated astroglia were mainly depicted at the border zone in terms of a glial scar formation. Early treatment with pigment epithelium-derived factor (PEDF) resulted in an attenuation of the usually up-regulated collagen IV-immunoreactivity. However, glial activation was not influenced by treatment with PEDF or the epidermal growth factor (EGF). In conclusion, these data on ischemia-induced cellular reactions within the NVU might help to develop treatments addressing the transition from injury towards regeneration. Thereby, the integrity of the vasculature in close relation to neighboring structures like astrocytes appears as a promising target.
Conflict of interest statement
Figures

References
-
- Donnan GA, Fisher M, Macleod M, Davis SM. Stroke. Lancet. 2008; 371: 1612–1623. doi: 10.1016/S0140-6736(08)60694-7 - DOI - PubMed
-
- Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, et al. Executive Summary: Heart Disease and Stroke Statistics– 2016 Update: A Report From the American Heart Association. Circulation. 2016; 133: 447–454. doi: 10.1161/CIR.0000000000000366 - DOI - PubMed
-
- Hacke W, Kaste M, Bluhmki E, Brozman M, Dávalos A, Guidetti D, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008; 359: 1317–1329. doi: 10.1056/NEJMoa0804656 - DOI - PubMed
-
- Friedrich B, Gawlitza M, Schob S, Hobohm C, Raviolo M, Hoffmann KT, et al. Distance to thrombus in acute middle cerebral artery occlusion: a predictor of outcome after intravenous thrombolysis for acute ischemic stroke. Stroke. 2015; 46: 692–696. doi: 10.1161/STROKEAHA.114.008454 - DOI - PubMed
-
- Heiss WD. Malignant MCA Infarction: Pathophysiology and Imaging for Early Diagnosis and Management Decisions. Cerebrovasc Dis. 2016; 41: 1–7. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

