No-touch radiofrequency ablation using multiple electrodes: An in vivo comparison study of switching monopolar versus switching bipolar modes in porcine livers
- PMID: 28445542
- PMCID: PMC5405985
- DOI: 10.1371/journal.pone.0176350
No-touch radiofrequency ablation using multiple electrodes: An in vivo comparison study of switching monopolar versus switching bipolar modes in porcine livers
Abstract
Objective: To evaluate the in vivo technical feasibility, efficiency, and safety of switching bipolar (SB) and switching monopolar (SM) radiofrequency ablation (RFA) as a no-touch ablation technique in the porcine liver.
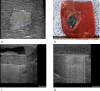
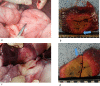
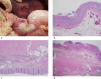
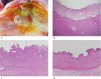
Materials and methods: The animal care and use committee approved this animal study and 16 pigs were used in two independent experiments. In the first experiment, RFA was performed on 2-cm tumor mimickers in the liver using a no-touch technique in the SM mode (2 groups, SM1: 10 minutes, n = 10; SM2: 15 minutes, n = 10) and SB-mode (1 group, SB: 10 minutes, n = 10). The technical success with sufficient safety margins, creation of confluent necrosis, ablation size, and distance between the electrode and ablation zone margin (DEM), were compared between groups. In the second experiment, thermal injury to the adjacent anatomic organs was compared between SM-RFA (15 minutes, n = 13) and SB-RFA modes (10 minutes, n = 13).
Results: The rates of the technical success and the creation of confluent necrosis were higher in the SB group than in the SM1 groups (100% vs. 60% and 90% vs. 40%, both p < 0.05). The ablation volume in the SM2 group was significantly larger than that in the SB group (59.2±18.7 cm3 vs. 39.8±9.7 cm3, p < 0.05), and the DEM in the SM2 group was also larger than that in the SB group (1.39±0.21 cm vs. 1.07±0.10 cm, p < 0.05). In the second experiment, the incidence of thermal injury to the adjacent organs and tissues in the SB group (23.1%, 3/13) was significantly lower than that in the SM group (69.2%, 8/13) (p = 0.021).
Conclusion: SB-RFA was more advantageous for a no-touch technique for liver tumors, showing the potential of a better safety profile than SM-RFA.
Conflict of interest statement
Figures


Similar articles
-
No-Touch Radiofrequency Ablation: A Comparison of Switching Bipolar and Switching Monopolar Ablation in Ex Vivo Bovine Liver.Korean J Radiol. 2017 Mar-Apr;18(2):279-288. doi: 10.3348/kjr.2017.18.2.279. Epub 2017 Feb 7. Korean J Radiol. 2017. PMID: 28246508 Free PMC article.
-
Switching bipolar hepatic radiofrequency ablation using internally cooled wet electrodes: comparison with consecutive monopolar and switching monopolar modes.Br J Radiol. 2015 Jun;88(1050):20140468. doi: 10.1259/bjr.20140468. Epub 2015 Apr 15. Br J Radiol. 2015. PMID: 25873479 Free PMC article.
-
Switching monopolar radiofrequency ablation technique using multiple, internally cooled electrodes and a multichannel generator: ex vivo and in vivo pilot study.Invest Radiol. 2007 Mar;42(3):163-71. doi: 10.1097/01.rli.0000252495.44818.b3. Invest Radiol. 2007. PMID: 17287646
-
Recent Advances in the Image-Guided Tumor Ablation of Liver Malignancies: Radiofrequency Ablation with Multiple Electrodes, Real-Time Multimodality Fusion Imaging, and New Energy Sources.Korean J Radiol. 2018 Jul-Aug;19(4):545-559. doi: 10.3348/kjr.2018.19.4.545. Epub 2018 Jun 14. Korean J Radiol. 2018. PMID: 29962861 Free PMC article. Review.
-
Radiofrequency ablation of primary and metastatic hepatic malignancies.Int J Clin Oncol. 2002 Apr;7(2):72-81. doi: 10.1007/s101470200010. Int J Clin Oncol. 2002. PMID: 12018113 Review.
Cited by
-
No-Touch Radiofrequency Ablation for Early Hepatocellular Carcinoma: 2023 Korean Society of Image-Guided Tumor Ablation Guidelines.Korean J Radiol. 2023 Aug;24(8):719-728. doi: 10.3348/kjr.2023.0423. Korean J Radiol. 2023. PMID: 37500573 Free PMC article. Review.
-
Influence of interapplicator distance on multibipolar radiofrequency ablation during physiological and interrupted liver perfusion in an in vivo porcine model.Sci Rep. 2020 Oct 1;10(1):16210. doi: 10.1038/s41598-020-71512-x. Sci Rep. 2020. PMID: 33004845 Free PMC article.
-
Review of Radiofrequency Ablation in Tonsillectomy.Indian J Otolaryngol Head Neck Surg. 2022 Dec;74(Suppl 3):5008-5011. doi: 10.1007/s12070-021-02626-5. Epub 2021 May 17. Indian J Otolaryngol Head Neck Surg. 2022. PMID: 36742542 Free PMC article.
-
Intra-articular hip joint osteoid osteoma: Challenging diagnosis and percutaneous radiofrequency ablation treatment.Radiol Case Rep. 2021 Aug 26;16(11):3315-3320. doi: 10.1016/j.radcr.2021.07.072. eCollection 2021 Nov. Radiol Case Rep. 2021. PMID: 34484539 Free PMC article.
-
Computed Tomography-Guided Percutaneous Radiofrequency Ablation in Older Adults With Early-Stage Peripheral Lung Cancer: A Retrospective Cohort Study.Cancer Control. 2022 Jan-Dec;29:10732748211070702. doi: 10.1177/10732748211070702. Cancer Control. 2022. PMID: 35076322 Free PMC article.
References
-
- Byrne TJ, Rakela J. Loco-regional therapies for patients with hepatocellular carcinoma awaiting liver transplantation: Selecting an optimal therapy. World J Transplant. 2016;6(2):306–13. PubMed Central PMCID: PMCPMC4919734. doi: 10.5500/wjt.v6.i2.306 - DOI - PMC - PubMed
-
- Bruix J, Sherman M, American Association for the Study of Liver D. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53(3):1020–2. PubMed Central PMCID: PMCPMC3084991. doi: 10.1002/hep.24199 - DOI - PMC - PubMed
-
- European Association For The Study Of The L, European Organisation For R, Treatment Of C. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56(4):908–43. doi: 10.1016/j.jhep.2011.12.001 - DOI - PubMed
-
- Salhab M, Canelo R. An overview of evidence-based management of hepatocellular carcinoma: a meta-analysis. J Cancer Res Ther. 2011;7(4):463–75. doi: 10.4103/0973-1482.92023 - DOI - PubMed
-
- Wu YZ, Li B, Wang T, Wang SJ, Zhou YM. Radiofrequency ablation vs hepatic resection for solitary colorectal liver metastasis: a meta-analysis. World J Gastroenterol. 2011;17(36):4143–8. PubMed Central PMCID: PMCPMC3203368. doi: 10.3748/wjg.v17.i36.4143 - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

