NLRP6 Protects Il10-/- Mice from Colitis by Limiting Colonization of Akkermansia muciniphila
- PMID: 28445725
- PMCID: PMC5528001
- DOI: 10.1016/j.celrep.2017.03.080
NLRP6 Protects Il10-/- Mice from Colitis by Limiting Colonization of Akkermansia muciniphila
Erratum in
-
NLRP6 Protects Il10-/- Mice from Colitis by Limiting Colonization of Akkermansia muciniphila.Cell Rep. 2017 Jun 6;19(10):2174. doi: 10.1016/j.celrep.2017.05.074. Cell Rep. 2017. PMID: 28591587 No abstract available.
Abstract
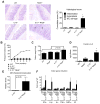
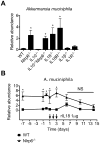
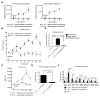
Dysfunction in host immune responses and pathologic alterations in the gut microbiota, referred to as dysbiosis, can both contribute to the development of inflammatory bowel disease (IBD). However, it remains unclear how specific changes in host immunity or the microbiota cause disease. We previously demonstrated that the loss of the innate immune receptor NLRP6 in mice resulted in impaired production of interleukin-18 (IL-18) and increased susceptibility to epithelial-induced injury. Here, we show that NLRP6 is important for suppressing the development of spontaneous colitis in the Il10-/- mice model of IBD and that NLRP6 deficiency results in the enrichment of Akkermansia muciniphila. A. muciniphila was sufficient for promoting intestinal inflammation in both specific-pathogen-free and germ-free Il10-/- mice. Our results demonstrate that A. muciniphila can act as a pathobiont to promote colitis in a genetically susceptible host and that NLRP6 is a key regulator of its abundance.
Keywords: Akkermansia; IL-10; Microbiota; NLRP6; NLRs; colitis; dysbiosis; inflammation; intestine; pathobiont.
Copyright © 2017 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors have declared no conflicts of interest.
Figures




References
-
- Couturier-Maillard A, Secher T, Rehman A, Normand S, De Arcangelis A, Haesler R, Huot L, Grandjean T, Bressenot A, Delanoye-Crespin A, et al. NOD2-mediated dysbiosis predisposes mice to transmissible colitis and colorectal cancer. The Journal of clinical investigation. 2013;123:700–711. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

