MiR-148a increases glioma cell migration and invasion by downregulating GADD45A in human gliomas with IDH1 R132H mutations
- PMID: 28445981
- PMCID: PMC5421935
- DOI: 10.18632/oncotarget.15867
MiR-148a increases glioma cell migration and invasion by downregulating GADD45A in human gliomas with IDH1 R132H mutations
Abstract
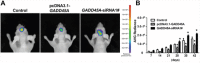
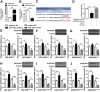
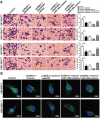
High-grade gliomas are severe tumors with poor prognosis. An R132H mutation in the isocitrate dehydrogenase (IDH1) gene prolongs the life of glioma patients. In this study, we investigated which genes are differentially regulated in IDH1 wild type (IDH1WT) or IDH1 R132H mutation (IDH1R132H) glioblastoma cells. Growth arrest and DNA-damage-inducible protein (GADD45A) was downregulated and microRNA 148a (miR-148a) was upregulated in in IDH1R132H human glioblastomas tissues. The relationship between GADD45A and miR-148a is unknown. In vitro experiments showed that GADD45A negatively regulates IDH1R132H glioma cell proliferation, migration, and invasion, and neurosphere formation in IDH1R132H glioblastoma stem cells (GSC). In addition, a human orthotopic xenograft mouse model showed that GADD45A reduced tumorigenesis in vivo. Our findings demonstrated that miR-148a promotes glioma cell invasion and tumorigenesis by downregulating GADD45A. Our findings provide novel insights into how GADD45A is downregulated by miR-148a in IDH1R132H glioma and may help to identify therapeutic targets for the effective treatment of high-grade glioma.
Keywords: GADD45A; invasion; miR-148a; migration; β-catenin.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures








References
-
- Chen R, Cohen AL, Colman H. Targeted Therapeutics in Patients With High-Grade Gliomas: Past, Present, and Future. Curr Treat Options Oncol. 2016;17:42. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

