Signaling by Antibodies: Recent Progress
- PMID: 28446061
- PMCID: PMC5613280
- DOI: 10.1146/annurev-immunol-051116-052433
Signaling by Antibodies: Recent Progress
Abstract
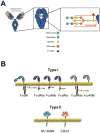
IgG antibodies mediate a diversity of immune functions by coupling of antigen specificity through the Fab domain to signal transduction via Fc-Fc receptor interactions. Indeed, balanced IgG signaling through type I and type II Fc receptors is required for the control of proinflammatory, anti-inflammatory, and immunomodulatory processes. In this review, we discuss the mechanisms that govern IgG-Fc receptor interactions, highlighting the diversity of Fc receptor-mediated effector functions that regulate immunity and inflammation as well as determine susceptibility to infection and autoimmunity and responsiveness to antibody-based therapeutics and vaccines.
Keywords: Fc receptors; IgG; effector function; immunity; immunomodulation; inflammation.
Figures
References
-
- Nimmerjahn F, Ravetch JV. Divergent immunoglobulin g subclass activity through selective Fc receptor binding. Science. 2005;310:1510–2. - PubMed
-
- Ferrara C, Grau S, Jäger C, Sondermann P, Brünker P, Waldhauer I, Hennig M, Ruf A, Rufer AC, Stihle M, Umaña P, Benz J. Unique carbohydrate-carbohydrate interactions are required for high affinity binding between FcgammaRIII and antibodies lacking core fucose. Proc Natl Acad Sci U S A. 2011;108:12669–74. - PMC - PubMed
-
- de Man YA, Dolhain RJ, Hazes JM. Disease activity or remission of rheumatoid arthritis before, during and following pregnancy. Curr Opin Rheumatol. 2014;26:329–33. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

