Effects of escitalopram and paroxetine on mTORC1 signaling in the rat hippocampus under chronic restraint stress
- PMID: 28446154
- PMCID: PMC5405541
- DOI: 10.1186/s12868-017-0357-0
Effects of escitalopram and paroxetine on mTORC1 signaling in the rat hippocampus under chronic restraint stress
Abstract
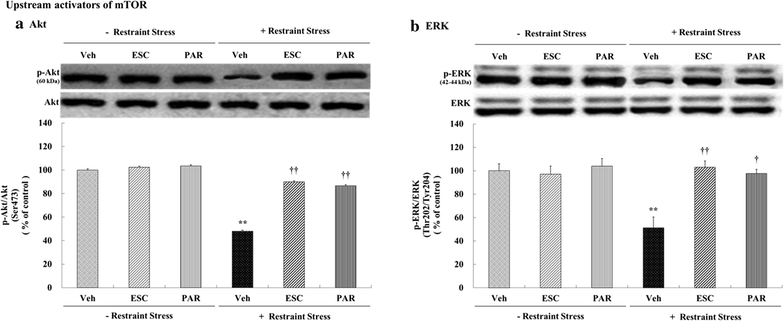
Background: Recent studies have suggested that the activation of mammalian target of rapamycin (mTOR) signaling may be related to antidepressant action. Therefore, the present study evaluated whether antidepressant drugs would exert differential effects on mTOR signaling in the rat hippocampus under conditions of chronic restraint stress. Male Sprague-Dawley rats were subjected to restraint stress for 6 h/days for 21 days with either escitalopram (10 mg/kg) or paroxetine (10 mg/kg) administered after the chronic stress procedure. Western blot analyses were used to assess changes in the levels of phospho-Ser2448-mTOR, phospho-Thr37/46-4E-BP-1, phospho-Thr389-p70S6 K, phospho-Ser422-eIF4B, phospho-Ser240/244-S6, phospho-Ser473-Akt, and phospho-Thr202/Tyr204-ERK in the hippocampus.
Results: Chronic restraint stress significantly decreased the levels of phospho-mTOR complex 1 (mTORC1), phospho-4E-BP-1, phospho-p70S6 K, phospho-eIF4B, phospho-S6, phospho-Akt, and phospho-ERK (p < 0.05); the administration of escitalopram and paroxetine increased the levels of all these proteins (p < 0.05 or 0.01). Additionally, chronic restraint stress reduced phospho-mTORC1 signaling activities in general, while escitalopram and paroxetine prevented these changes in phospho-mTORC1 signaling activities.
Conclusion: These findings provide further data that contribute to understanding the possible relationships among mTOR activity, stress, and antidepressant drugs.
Keywords: Antidepressants; Chronic restraint stress; Hippocampus; Neuroplasticity; mTOR signaling.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

