Cooperation of neurotrophin receptor TrkB and Her2 in breast cancer cells facilitates brain metastases
- PMID: 28446206
- PMCID: PMC5406906
- DOI: 10.1186/s13058-017-0844-3
Cooperation of neurotrophin receptor TrkB and Her2 in breast cancer cells facilitates brain metastases
Abstract
Background: Patients with primary breast cancer that is positive for human epidermal growth factor receptor 2 (Her2+) have a high risk of developing metastases in the brain. Despite gains with systemic control of Her2+ disease using molecular therapies, brain metastases remain recalcitrant to therapeutic discovery. The clinical predilection of Her2+ breast cancer cells to colonize the brain likely relies on paracrine mechanisms. The neural niche poses unique selection pressures, and neoplastic cells that utilize the brain microenvironment may have a survival advantage.
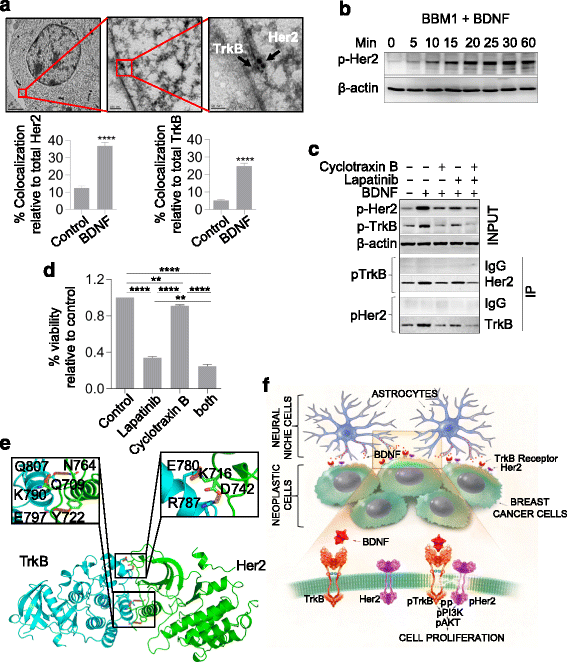
Methods: Tropomyosin-related kinase B (TrkB), Her2, and downstream targets were analyzed in primary breast cancer, breast-to-brain metastasis (BBM) tissues, and tumor-derived cell lines using quantitative real-time PCR, western blot, and immunohistochemical assessment. TrkB function on BBM was confirmed with intracranial, intracardiac, or mammary fat pad xenografts in non-obese diabetic/severe combined immunodeficiency mice. The function of brain-derived neurotrophic factor (BDNF) on cell proliferation and TrkB/Her2 signaling and interactions were confirmed using selective shRNA knockdown and selective inhibitors. The physical interaction of Her2-TrkB was analyzed using electron microscopy, co-immunoprecipitation, and in silico analysis. Dual targeting of Her2 and TrkB was analyzed using clinically utilized treatments.
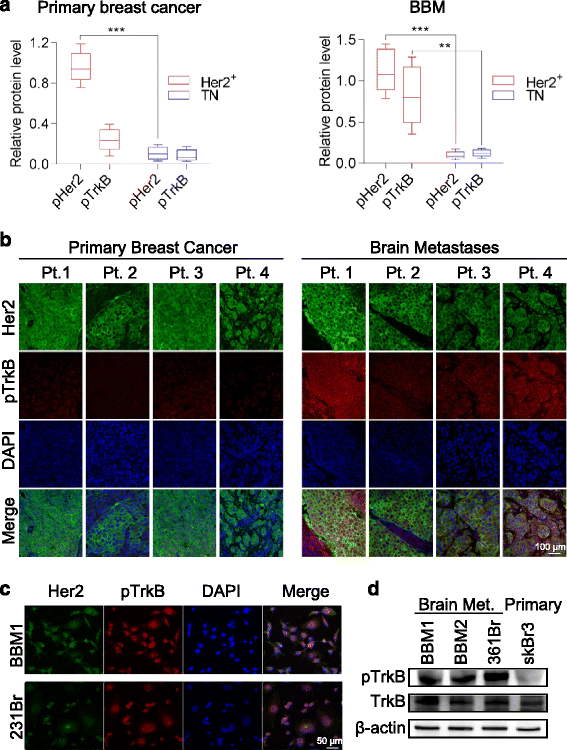
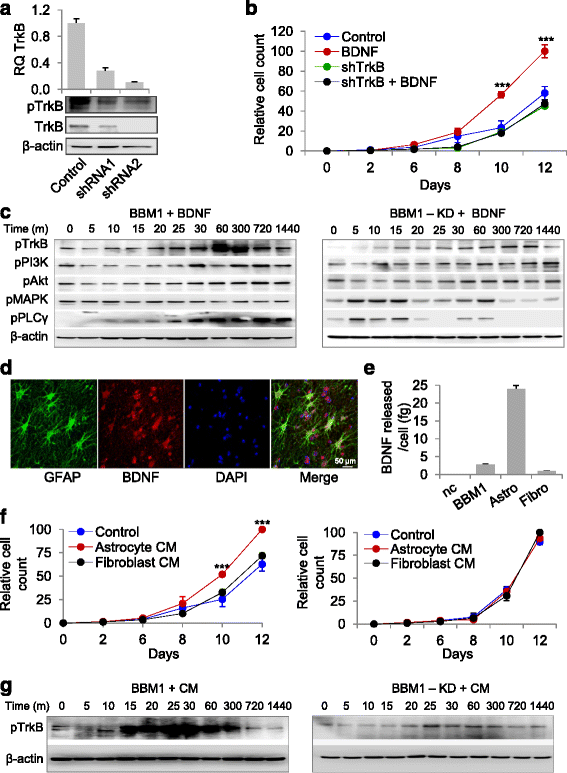
Results: We observed that patient tissues and cell lines derived from Her2+ human BBM displayed increased activation of TrkB, a neurotrophin receptor. BDNF, an extracellular neurotrophin, with roles in neuronal maturation and homeostasis, specifically binds to TrkB. TrkB knockdown in breast cancer cells led to decreased frequency and growth of brain metastasis in animal models, suggesting that circulating breast cancer cells entering the brain may take advantage of paracrine BDNF-TrkB signaling for colonization. In addition, we investigated a possible interaction between TrkB and Her2 receptors on brain metastatic breast cancer cells, and found that BDNF phosphorylated both its cognate TrkB receptor and the Her2 receptor in brain metastatic breast cancer cells.
Conclusion: Collectively, our findings suggest that heterodimerization of Her2 and TrkB receptors gives breast cancer cells a survival advantage in the brain and that dual inhibition of these receptors may hold therapeutic potential.
Keywords: Astrocyte; Brain-derived neurotrophic factor (BDNF); Breast cancer brain metastasis; Cyclotraxin B; Epidermal growth factor receptor 2 (Her2+); Glial cells; Lapatinib; Neural microenvironment; Neurotrophic factor; Neurotrophins; Tropomyosin-related kinase B (TrkB).
Figures

References
-
- Palmieri D. An introduction to brain metastasis. In: Palmieri D, editor. Central nervous system metastasis, the biological basis, and clinical considerations. Dordrecht, The Netherlands: Springer; 2012. pp. 1–13.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

