A Novel α-Calcitonin Gene-Related Peptide Analogue Protects Against End-Organ Damage in Experimental Hypertension, Cardiac Hypertrophy, and Heart Failure
- PMID: 28446517
- PMCID: PMC5519346
- DOI: 10.1161/CIRCULATIONAHA.117.028388
A Novel α-Calcitonin Gene-Related Peptide Analogue Protects Against End-Organ Damage in Experimental Hypertension, Cardiac Hypertrophy, and Heart Failure
Abstract
Background: Research into the therapeutic potential of α-calcitonin gene-related peptide (α-CGRP) has been limited because of its peptide nature and short half-life. Here, we evaluate whether a novel potent and long-lasting (t½ ≥7 hours) acylated α-CGRP analogue (αAnalogue) could alleviate and reverse cardiovascular disease in 2 distinct murine models of hypertension and heart failure in vivo.
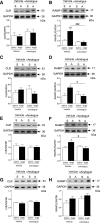
Methods: The ability of the αAnalogue to act selectively via the CGRP pathway was shown in skin by using a CGRP receptor antagonist. The effect of the αAnalogue on angiotensin II-induced hypertension was investigated over 14 days. Blood pressure was measured by radiotelemetry. The ability of the αAnalogue to modulate heart failure was studied in an abdominal aortic constriction model of murine cardiac hypertrophy and heart failure over 5 weeks. Extensive ex vivo analysis was performed via RNA analysis, Western blot, and histology.
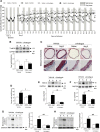
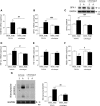
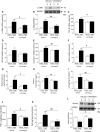
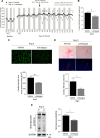
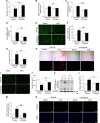
Results: The angiotensin II-induced hypertension was attenuated by cotreatment with the αAnalogue (50 nmol·kg-1·d-1, SC, at a dose selected for lack of long-term hypotensive effects at baseline). The αAnalogue protected against vascular, renal, and cardiac dysfunction, characterized by reduced hypertrophy and biomarkers of fibrosis, remodeling, inflammation, and oxidative stress. In a separate study, the αAnalogue reversed angiotensin II-induced hypertension and associated vascular and cardiac damage. The αAnalogue was effective over 5 weeks in a murine model of cardiac hypertrophy and heart failure. It preserved heart function, assessed by echocardiography, while protecting against adverse cardiac remodeling and apoptosis. Moreover, treatment with the αAnalogue was well tolerated with neither signs of desensitization nor behavioral changes.
Conclusions: These findings, in 2 distinct models, provide the first evidence for the therapeutic potential of a stabilized αAnalogue, by mediating (1) antihypertensive effects, (2) attenuating cardiac remodeling, and (3) increasing angiogenesis and cell survival to protect against and limit damage associated with the progression of cardiovascular diseases. This indicates the therapeutic potential of the CGRP pathway and the possibility that this injectable CGRP analogue may be effective in cardiac disease.
Keywords: heart failure; hypertension; inflammation; oxidative stress; receptors, calcitonin gene-related peptide.
© 2017 The Authors.
Figures


Comment in
-
Calcitonin Gene-Related Peptide Receptor Agonism: A Double-Edged Sword?Circulation. 2017 Jul 25;136(4):384-387. doi: 10.1161/CIRCULATIONAHA.117.028955. Circulation. 2017. PMID: 28739811 No abstract available.
-
Letter by Tsuda Regarding Article, "A Novel α-Calcitonin Gene-Related Peptide Analogue Protects Against End-Organ Damage in Experimental Hypertension, Cardiac Hypertrophy, and Heart Failure".Circulation. 2018 Mar 13;137(11):1198-1199. doi: 10.1161/CIRCULATIONAHA.117.030714. Circulation. 2018. PMID: 29530896 No abstract available.
-
Letter by Jin-shan and Xue-bin Regarding Article, "A Novel α-Calcitonin Gene-Related Peptide Analogue Protects Against End-Organ Damage in Experimental Hypertension, Cardiac Hypertrophy, and Heart Failure".Circulation. 2018 Mar 13;137(11):1200. doi: 10.1161/CIRCULATIONAHA.117.031504. Circulation. 2018. PMID: 29530897 No abstract available.
-
Response by Aubdool et al to Letters Regarding Article, "A Novel α-Calcitonin Gene-Related Peptide Analogue Protects Against End-Organ Damage in Experimental Hypertension, Cardiac Hypertrophy, and Heart Failure".Circulation. 2018 Mar 13;137(11):1201-1202. doi: 10.1161/CIRCULATIONAHA.117.031848. Circulation. 2018. PMID: 29530898 No abstract available.
References
-
- Diener HC, Charles A, Goadsby PJ, Holle D. New therapeutic approaches for the prevention and treatment of migraine. Lancet Neurol. 2015;14:1010–1022. doi: 10.1016/S1474-4422(15)00198-2. - PubMed
-
- Fujioka S, Sasakawa O, Kishimoto H, Tsumura K, Morii H. The antihypertensive effect of calcitonin gene-related peptide in rats with norepinephrine- and angiotensin II-induced hypertension. J Hypertens. 1991;9:175–179. - PubMed
-
- Hobara N, Gessei-Tsutsumi N, Goda M, Takayama F, Akiyama S, Kurosaki Y, Kawasaki H. Long-term inhibition of angiotensin prevents reduction of periarterial innervation of calcitonin gene-related peptide (CGRP)-containing nerves in spontaneously hypertensive rats. Hypertens Res. 2005;28:465–474. doi: 10.1291/hypres.28.465. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

