Comparison of Ticagrelor Pharmacokinetics and Pharmacodynamics in STEMI and NSTEMI Patients (PINPOINT): protocol for a prospective, observational, single-centre study
- PMID: 28446521
- PMCID: PMC5541360
- DOI: 10.1136/bmjopen-2016-013218
Comparison of Ticagrelor Pharmacokinetics and Pharmacodynamics in STEMI and NSTEMI Patients (PINPOINT): protocol for a prospective, observational, single-centre study
Abstract
Introduction: The most common classification of acute myocardial infarction (AMI) is based on electrocardiographic findings and distinguishes ST-elevation myocardial infarction (STEMI) and non-ST-elevation myocardial infarction (NSTEMI). Both types of AMI differ concerning their epidemiology, clinical approach and early outcomes. Ticagrelor is a P2Y12 receptor inhibitor, constituting the first-line treatment for STEMI and NSTEMI. According to available data, STEMI may be associated with lower plasma concentration of ticagrelor in the first hours of AMI, but currently there are no studies directly comparing ticagrelor pharmacokinetics or antiplatelet effect in patients with STEMI versus NSTEMI.
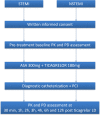
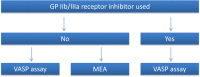
Methods and analysis: The PINPOINT study is a phase IV, single-centre, investigator-initiated, prospective, observational study designed to compare the pharmacokinetics and pharmacodynamics of ticagrelor in patients with STEMI and NSTEMI assigned to the invasive strategy of treatment. Based on an internal pilot study, the trial is expected to include at least 23 patients with each AMI type. All subjects will receive a 180 mg loading dose of ticagrelor. The primary end point of the study is the area under the plasma concentration-time curve (AUC(0-6)) for ticagrelor during the first 6 hours after the loading dose. Secondary end points include various pharmacokinetic features of ticagrelor and its active metabolite (AR-C124910XX), and evaluation of platelet reactivity by the vasodilator-stimulated phosphoprotein assay and multiple electrode aggregometry. Blood samples for the pharmacokinetic and pharmacodynamic assessment will be obtained at pretreatment, 30 min, 1, 2, 3, 4, 6 and 12 hours post-ticagrelor loading dose.
Ethics and dissemination: The study received approval from the Local Ethics Committee (Komisja Bioetyczna Uniwersytetu Mikołaja Kopernika w Toruniu przy Collegium Medicum im. Ludwika Rydygiera w Bydgoszczy; approval reference number KB 617/2015). The study results will be disseminated through conference presentations and peer-reviewed journals.
Trial registration number: NCT02602444; Pre-results.
Keywords: NSTEMI; STEMI; pharmacodynamics; pharmacokinetics; ticagrelor.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://www.bmj.com/company/products-services/rights-and-licensing/.
Conflict of interest statement
Competing interests: JK received a consulting fee from AstraZeneca. MK received honoraria for lectures from AstraZeneca. All other authors have reported no relationships relevant to the contents of this paper.
Figures
References
-
- Roffi M, Patrono C, Collet JP, et al. . 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: Task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC). Eur Heart J 2016;37:267–315. 10.1093/eurheartj/ehv320 - DOI - PubMed
-
- Kubica J. The optimal antiplatelet treatment in an emergency setting. Folia Med Copernicana 2014;2:73–6.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
