Induction of Adaptive Immunity Leads to Nigrostriatal Disease Progression in MPTP Mouse Model of Parkinson's Disease
- PMID: 28446566
- PMCID: PMC5467696
- DOI: 10.4049/jimmunol.1700149
Induction of Adaptive Immunity Leads to Nigrostriatal Disease Progression in MPTP Mouse Model of Parkinson's Disease
Abstract
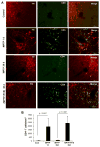
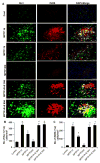
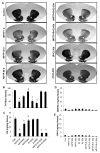
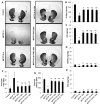
Although the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) mouse model is the most widely used animal model for Parkinson's disease (PD), it is known that nigrostriatal pathologies do not persist in the acute MPTP mouse model. This study highlights the importance of adaptive immunity in driving persistent and progressive disease in acute MPTP-intoxicated mice. Although marked infiltration of T cells into the nigra was found on 1 d of MPTP insult, T cell infiltration decreased afterward, becoming normal on 30 d of insult. Interestingly, twice-weekly supplementation of RANTES and eotaxin, chemokines that are involved in T cell trafficking, drove continuous T cell infiltration to the nigra and incessant glial inflammation. Supplementation of RANTES and eotaxin was also associated with the induction of nigral α-synuclein pathology, persistent loss of dopaminergic neurons and striatal neurotransmitters, and continuous impairment of motor functions in MPTP-intoxicated mice. In contrast, supplementation of TNF-α and IL-1β, widely studied proinflammatory cytokines, did not induce persistent disease in MPTP-insulted mice. Our results suggest that induction of adaptive immunity by RANTES and eotaxin could hold the key for driving persistent nigrostriatal pathologies in the MPTP mouse model, and that targeting these factors may halt disease progression in PD patients.
Copyright © 2017 by The American Association of Immunologists, Inc.
Figures







References
-
- Vila M, Przedborski S. Genetic clues to the pathogenesis of Parkinson’s disease. Nat Med. 2004;(10 Suppl):S58–62. - PubMed
-
- Olanow CW, Tatton WG. Etiology and pathogenesis of Parkinson’s disease. Annu Rev Neurosci. 1999;22:123–144. - PubMed
-
- Dauer W, Przedborski S. Parkinson’s disease: mechanisms and models. Neuron. 2003;39:889–909. - PubMed
-
- Kordower JH, Emborg ME, Bloch J, Ma SY, Chu Y, Leventhal L, McBride J, Chen EY, Palfi S, Roitberg BZ, Brown WD, Holden JE, Pyzalski R, Taylor MD, Carvey P, Ling Z, Trono D, Hantraye P, Deglon N, Aebischer P. Neurodegeneration prevented by lentiviral vector delivery of GDNF in primate models of Parkinson’s disease. Science. 2000;290:767–773. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

