Diagnostic Value of Galactomannan Antigen Test in Serum and Bronchoalveolar Lavage Fluid Samples from Patients with Nonneutropenic Invasive Pulmonary Aspergillosis
- PMID: 28446576
- PMCID: PMC5483917
- DOI: 10.1128/JCM.00345-17
Diagnostic Value of Galactomannan Antigen Test in Serum and Bronchoalveolar Lavage Fluid Samples from Patients with Nonneutropenic Invasive Pulmonary Aspergillosis
Abstract
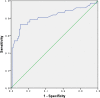
The objective of this study was to compare the diagnostic value of galactomannan (GM) detection in bronchoalveolar lavage fluid (BALF) and serum samples from nonneutropenic patients with invasive pulmonary aspergillosis (IPA) and determine the optimal BALF GM cutoff value for pulmonary aspergillosis. GM detection in BALF and serum samples was performed by enzyme-linked immunosorbent assay (ELISA) in 128 patients with clinically suspected nonneutropenic pulmonary aspergillosis between June 2014 and June 2016. On the basis of the clinical and pathological diagnoses, 8 patients were excluded because their diagnosis was uncertain. The remaining 120 patients were diagnosed with either IPA (n = 37), community-acquired pneumonia (CAP; n = 59), noninfectious diseases (n = 19), or tuberculosis (n = 5). At a cutoff optical density index (ODI) value of ≥0.5, the sensitivity of BALF GM detection was much higher than that of serum GM detection (75.68% versus 37.84%; P = 0.001), but there was no significant difference between their specificities (80.72% versus 87.14%; P = 0.286). At a cutoff value of ≥1.0, the sensitivity of BALF GM detection was still much higher than that of serum GM detection (64.86% versus 24.32%; P < 0.001), and their specificities were similar (90.36% versus 95.71%; P = 0.202). Receiver operating characteristic (ROC) curve analysis showed that when the BALF GM detection cutoff value was 0.7, its diagnostic value for pulmonary aspergillosis was optimized, and the sensitivity and specificity reached 72.97% and 89.16%, respectively. BALF GM detection was valuable for the diagnosis of IPA in nonneutropenic patients, and its diagnostic value was superior to that of serum GM detection. The optimal BALF GM cutoff value was 0.7.
Keywords: bronchoalveolar lavage fluid; galactomannan antigen; invasive pulmonary aspergillosis; nonneutropenic patients.
Copyright © 2017 American Society for Microbiology.
Figures
References
-
- Lopez-Medrano F, Silva JT, Fernandez-Ruiz M, Carver PL, van Delden C, Merino E, Pérez-Saez MJ, Montero M, Coussement J, de Abreu Mazzolin M, Cervera C, Santos L, Sabé N, Scemla A, Cordero E, Cruzado-Vega L, Martín-Moreno PL, Len Ó, Rudas E, de León AP, Arriola M, Lauzurica R, David M, González-Rico C, Henríquez-Palop F, Fortún J, Nucci M, Manuel O, Paño-Pardo JR, Montejo M, Muñoz P, Sánchez-Sobrino B, Mazuecos A, Pascual J, Horcajada JP, Lecompte T, Lumbreras C, Moreno A, Carratalà J, Blanes M, Hernández D, Hernández-Méndez EA, Fariñas MC, Perelló-Carrascosa M, Morales JM, Andrés A, Aguado JM. 2016. Risk factors associated with early invasive pulmonary aspergillosis in kidney transplant recipients: results from a multinational matched case-control study. Am J Transplant 16:2148–2157. doi: 10.1111/ajt.13735. - DOI - PubMed
-
- De Pauw B, Walsh TJ, Donnelly JP, Stevens DA, Edwards JE, Calandra T, Pappas PG, Maertens J, Lortholary O, Kauffman CA, Denning DW, Patterson TF, Maschmeyer G, Bille J, Dismukes WE, Herbrecht R, Hope WW, Kibbler CC, Kullberg BJ, Marr KA, Muñoz P, Odds FC, Perfect JR, Restrepo A, Ruhnke M, Segal BH, Sobel JD, Sorrell TC, Viscoli C, Wingard JR, Zaoutis T, Bennett JE. 2008. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis 46:1813–1821. doi: 10.1086/588660. - DOI - PMC - PubMed
-
- Pagano L, Caira M, Candoni A, Offidani M, Fianchi L, Martino B, Pastore D, Picardi M, Bonini A, Chierichini A, Fanci R, Caramatti C, Invernizzi R, Mattei D, Mitra ME, Melillo L, Aversa F, Van Lint MT, Falcucci P, Valentini CG, Girmenia C, Nosari A. 2006. The epidemiology of fungal infections in patients with hematologic malignancies: the SEIFEM-2004 study. Haematologica 91:1068–1075. - PubMed
-
- Delsuc C, Cottereau A, Frealle E, Bienvenu AL, Dessein R, Jarraud S, Dumitrescu O, Le Maréchal M, Wallet F, Friggeri A, Argaud L, Rimmelé T, Nseir S, Ader F. 2015. Putative invasive pulmonary aspergillosis in critically ill patients with chronic obstructive pulmonary disease: a matched cohort study. Crit Care 19:421. doi: 10.1186/s13054-015-1140-1. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous