Human primary somatosensory cortex is differentially involved in vibrotaction and nociception
- PMID: 28446584
- PMCID: PMC5498736
- DOI: 10.1152/jn.00615.2016
Human primary somatosensory cortex is differentially involved in vibrotaction and nociception
Abstract
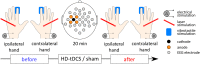
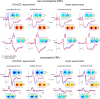
The role of the primary somatosensory cortex (S1) in vibrotaction is well established. In contrast, its involvement in nociception is still debated. Here we test whether S1 is similarly involved in the processing of nonnociceptive and nociceptive somatosensory input in humans by comparing the aftereffects of high-definition transcranial direct current stimulation (HD-tDCS) of S1 on the event-related potentials (ERPs) elicited by nonnociceptive and nociceptive somatosensory stimuli delivered to the ipsilateral and contralateral hands. Cathodal HD-tDCS significantly affected the responses to nonnociceptive somatosensory stimuli delivered to the contralateral hand: both early-latency ERPs from within S1 (N20 wave elicited by transcutaneous electrical stimulation of median nerve) and late-latency ERPs elicited outside S1 (N120 wave elicited by short-lasting mechanical vibrations delivered to index fingertip, thought to originate from bilateral operculo-insular and cingulate cortices). These results support the notion that S1 constitutes an obligatory relay for the cortical processing of nonnociceptive tactile input originating from the contralateral hemibody. Contrasting with this asymmetric effect of HD-tDCS on the responses to nonnociceptive somatosensory input, HD-tDCS over the sensorimotor cortex led to a bilateral and symmetric reduction of the magnitude of the N240 wave of nociceptive laser-evoked potentials elicited by stimulation of the hand dorsum. Taken together, our results demonstrate in humans a differential involvement of S1 in vibrotaction and nociception.NEW & NOTEWORTHY Whereas the role of the primary somatosensory cortex (S1) in vibrotaction is well established, its involvement in nociception remains strongly debated. By assessing, in healthy volunteers, the effect of high-definition transcranial direct current stimulation over S1, we demonstrate a differential involvement of S1 in vibrotaction and nociception.
Keywords: evoked potentials; nociception; primary somatosensory cortex; touch; transcranial direct current stimulation.
Copyright © 2017 the American Physiological Society.
Figures



Similar articles
-
Unmasking the obligatory components of nociceptive event-related brain potentials.J Neurophysiol. 2013 Nov;110(10):2312-24. doi: 10.1152/jn.00137.2013. Epub 2013 Aug 21. J Neurophysiol. 2013. PMID: 23966678
-
Theta burst stimulation applied over primary motor and somatosensory cortices produces analgesia unrelated to the changes in nociceptive event-related potentials.PLoS One. 2013 Aug 20;8(8):e73263. doi: 10.1371/journal.pone.0073263. eCollection 2013. PLoS One. 2013. PMID: 23977382 Free PMC article.
-
The primary somatosensory cortex and the insula contribute differently to the processing of transient and sustained nociceptive and non-nociceptive somatosensory inputs.Hum Brain Mapp. 2015 Nov;36(11):4346-4360. doi: 10.1002/hbm.22922. Epub 2015 Aug 7. Hum Brain Mapp. 2015. PMID: 26252509 Free PMC article.
-
Differential effects of cathodal transcranial direct current stimulation of prefrontal, motor and somatosensory cortices on cortical excitability and pain perception - a double-blind randomised sham-controlled study.Eur J Neurosci. 2015 Oct;42(7):2426-37. doi: 10.1111/ejn.13043. Epub 2015 Sep 12. Eur J Neurosci. 2015. PMID: 26275236 Clinical Trial.
-
Pain perception: is there a role for primary somatosensory cortex?Proc Natl Acad Sci U S A. 1999 Jul 6;96(14):7705-9. doi: 10.1073/pnas.96.14.7705. Proc Natl Acad Sci U S A. 1999. PMID: 10393884 Free PMC article. Review.
Cited by
-
Cold-evoked potentials in Fabry disease and polyneuropathy.Front Pain Res (Lausanne). 2024 May 15;5:1352711. doi: 10.3389/fpain.2024.1352711. eCollection 2024. Front Pain Res (Lausanne). 2024. PMID: 38812855 Free PMC article.
-
The amplitude of low frequency fluctuation and spontaneous brain activity alterations in age-related macular degeneration.Front Med (Lausanne). 2025 Jan 22;11:1507971. doi: 10.3389/fmed.2024.1507971. eCollection 2024. Front Med (Lausanne). 2025. PMID: 39911676 Free PMC article.
-
Distinct neocortical mechanisms underlie human SI responses to median nerve and laser-evoked peripheral activation.Imaging Neurosci (Camb). 2024 Feb 22;2:imag-2-00095. doi: 10.1162/imag_a_00095. eCollection 2024. Imaging Neurosci (Camb). 2024. PMID: 40800401 Free PMC article.
-
Transcutaneous auricular VNS applied to experimental pain: A paired behavioral and EEG study using thermonociceptive CO2 laser.PLoS One. 2021 Jul 12;16(7):e0254480. doi: 10.1371/journal.pone.0254480. eCollection 2021. PLoS One. 2021. PMID: 34252124 Free PMC article.
-
Somatosensory Gating Is Modulated by Anodal Transcranial Direct Current Stimulation.Front Neurosci. 2021 Sep 7;15:651253. doi: 10.3389/fnins.2021.651253. eCollection 2021. Front Neurosci. 2021. PMID: 34557064 Free PMC article.
References
-
- Allison T, McCarthy G, Wood CC, Darcey TM, Spencer DD, Williamson PD. Human cortical potentials evoked by stimulation of the median nerve. I. Cytoarchitectonic areas generating short-latency activity. J Neurophysiol 62: 694–710, 1989a. - PubMed
-
- Allison T, McCarthy G, Wood CC, Williamson PD, Spencer DD. Human cortical potentials evoked by stimulation of the median nerve. II. Cytoarchitectonic areas generating long-latency activity. J Neurophysiol 62: 711–722, 1989b. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

