Activation mechanism of a neuromodulator-gated pacemaker ionic current
- PMID: 28446585
- PMCID: PMC5511868
- DOI: 10.1152/jn.00743.2016
Activation mechanism of a neuromodulator-gated pacemaker ionic current
Abstract
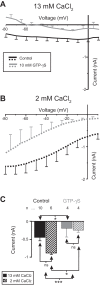
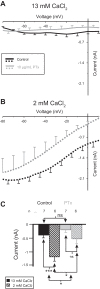
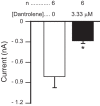
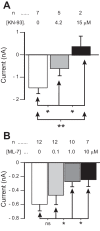
The neuromodulator-gated current (IMI) found in the crab stomatogastric ganglion is activated by neuromodulators that are essential to induce the rhythmic activity of the pyloric network in this system. One of these neuromodulators is also known to control the correlated expression of voltage-gated ionic currents in pyloric neurons, as well as synaptic plasticity and strength. Thus understanding the mechanism by which neuromodulator receptors activate IMI should provide insights not only into how oscillations are initiated but also into how other processes, and currents not directly activated by them, are regulated. To determine what specific signaling molecules are implicated in this process, we used a battery of agonists and antagonists of common signal transduction pathways. We found that the G protein inhibitor GDPβS and the G protein activator GTPγS significantly affect IMI amplitude, suggesting that its activation is mediated by G proteins. Interestingly, when using the more specific G protein blocker pertussis toxin, we observed the expected inhibition of IMI amplitude but, unexpectedly, in a calcium-dependent fashion. We also found that antagonists of calcium- and calmodulin-associated signaling significantly reduce IMI amplitude. In contrast, we found little evidence for the role of cyclic nucleotide signaling, phospholipase C (PLC), or kinases and phosphatases, except two calmodulin-dependent kinases. In sum, these results suggest that proctolin-induced IMI is mediated by a G protein whose pertussis toxin sensitivity is altered by external calcium concentration and appears to depend on intracellular calcium, calmodulin, and calmodulin-activated kinases. In contrast, we found no support for IMI being mediated by PLC signaling or cyclic nucleotides.NEW & NOTEWORTHY Neuronal rhythmic activity is generated by either network-based or cell-autonomous mechanisms. In the pyloric network of decapod crustaceans, the activation of a neuromodulator-gated pacemaker current is crucial for the generation of rhythmic activity. This current is activated by several neuromodulators, including peptides and acetylcholine, presumably via metabotropic receptors. We have previously demonstrated a novel extracellular calcium-sensitive voltage-dependence mechanism of this current. We presently report that the activation mechanism depends on intracellular and extracellular calcium-sensitive components.
Keywords: G proteins; calcium; calmodulin; pacemaker; signaling.
Copyright © 2017 the American Physiological Society.
Figures




Similar articles
-
Graded Transmission without Action Potentials Sustains Rhythmic Activity in Some But Not All Modulators That Activate the Same Current.J Neurosci. 2018 Oct 17;38(42):8976-8988. doi: 10.1523/JNEUROSCI.2632-17.2018. Epub 2018 Sep 5. J Neurosci. 2018. PMID: 30185461 Free PMC article.
-
Voltage Dependence of a Neuromodulator-Activated Ionic Current.eNeuro. 2016 May 12;3(2):ENEURO.0038-16.2016. doi: 10.1523/ENEURO.0038-16.2016. eCollection 2016 Mar-Apr. eNeuro. 2016. PMID: 27257619 Free PMC article.
-
Multiple peptides converge to activate the same voltage-dependent current in a central pattern-generating circuit.J Neurosci. 2000 Sep 15;20(18):6752-9. doi: 10.1523/JNEUROSCI.20-18-06752.2000. J Neurosci. 2000. PMID: 10995818 Free PMC article.
-
Neurophysiology of HCN channels: from cellular functions to multiple regulations.Prog Neurobiol. 2014 Jan;112:1-23. doi: 10.1016/j.pneurobio.2013.10.001. Epub 2013 Oct 29. Prog Neurobiol. 2014. PMID: 24184323 Review.
-
Molecular regulation and pharmacology of pacemaker channels.Curr Pharm Des. 2007;13(23):2338-49. doi: 10.2174/138161207781368729. Curr Pharm Des. 2007. PMID: 17692005 Review.
Cited by
-
Modulation by Neuropeptides with Overlapping Targets Results in Functional Overlap in Oscillatory Circuit Activation.J Neurosci. 2024 Jan 3;44(1):e1201232023. doi: 10.1523/JNEUROSCI.1201-23.2023. J Neurosci. 2024. PMID: 37968117 Free PMC article.
-
Modulation of Neural Microcircuits That Control Complex Dynamics in Olfactory Networks.Front Cell Neurosci. 2021 Jun 22;15:662184. doi: 10.3389/fncel.2021.662184. eCollection 2021. Front Cell Neurosci. 2021. PMID: 34239417 Free PMC article.
-
Convergent Comodulation Reduces Interindividual Variability of Circuit Output.eNeuro. 2024 Sep 10;11(9):ENEURO.0167-24.2024. doi: 10.1523/ENEURO.0167-24.2024. Print 2024 Sep. eNeuro. 2024. PMID: 39134416 Free PMC article.
-
Graded Transmission without Action Potentials Sustains Rhythmic Activity in Some But Not All Modulators That Activate the Same Current.J Neurosci. 2018 Oct 17;38(42):8976-8988. doi: 10.1523/JNEUROSCI.2632-17.2018. Epub 2018 Sep 5. J Neurosci. 2018. PMID: 30185461 Free PMC article.
-
The differential contribution of pacemaker neurons to synaptic transmission in the pyloric network of the Jonah crab, Cancer borealis.J Neurophysiol. 2019 Oct 1;122(4):1623-1633. doi: 10.1152/jn.00038.2019. Epub 2019 Aug 14. J Neurophysiol. 2019. PMID: 31411938 Free PMC article.
References
-
- Baines RA, Walther C, Hinton JM, Osborne RH, Konopińska D. Selective activity of a proctolin analogue reveals the existence of two receptor subtypes. J Neurophysiol 75: 2647–2650, 1996. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

