CDK4 protein is degraded by anaphase-promoting complex/cyclosome in mitosis and reaccumulates in early G1 phase to initiate a new cell cycle in HeLa cells
- PMID: 28446612
- PMCID: PMC5473219
- DOI: 10.1074/jbc.M116.773226
CDK4 protein is degraded by anaphase-promoting complex/cyclosome in mitosis and reaccumulates in early G1 phase to initiate a new cell cycle in HeLa cells
Abstract
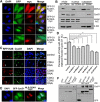
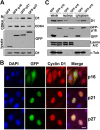
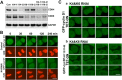
CDK4 regulates G1/S phase transition in the mammalian cell cycle by phosphorylating retinoblastoma family proteins. However, the mechanism underlying the regulation of CDK4 activity is not fully understood. Here, we show that CDK4 protein is degraded by anaphase-promoting complex/cyclosome (APC/C) during metaphase-anaphase transition in HeLa cells, whereas its main regulator, cyclin D1, remains intact but is sequestered in cytoplasm. CDK4 protein reaccumulates in the following G1 phase and shuttles between the nucleus and the cytoplasm to facilitate the nuclear import of cyclin D1. Without CDK4, cyclin D1 cannot enter the nucleus. Point mutations that disrupt CDK4 and cyclin D1 interaction impair the nuclear import of cyclin D1 and the activity of CDK4. RNAi knockdown of CDK4 also induces cytoplasmic retention of cyclin D1 and G0/G1 phase arrest of the cells. Collectively, our data demonstrate that CDK4 protein is degraded in late mitosis and reaccumulates in the following G1 phase to facilitate the nuclear import of cyclin D1 for activation of CKD4 to initiate a new cell cycle in HeLa cells.
Keywords: cell cycle; cyclin D1; cyclin-dependent kinase (CDK); protein degradation; retinoblastoma protein (pRb, RB).
© 2017 by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures




Similar articles
-
Critical role of cyclin D1 nuclear import in cardiomyocyte proliferation.Circ Res. 2003 Jan 10;92(1):e12-9. doi: 10.1161/01.res.0000049105.15329.1c. Circ Res. 2003. PMID: 12522130
-
APC/C prevents a noncanonical order of cyclin/CDK activity to maintain CDK4/6 inhibitor-induced arrest.Proc Natl Acad Sci U S A. 2024 Jul 23;121(30):e2319574121. doi: 10.1073/pnas.2319574121. Epub 2024 Jul 18. Proc Natl Acad Sci U S A. 2024. PMID: 39024113 Free PMC article.
-
Wogonin induces G1 phase arrest through inhibiting Cdk4 and cyclin D1 concomitant with an elevation in p21Cip1 in human cervical carcinoma HeLa cells.Biochem Cell Biol. 2009 Dec;87(6):933-42. doi: 10.1139/o09-060. Biochem Cell Biol. 2009. PMID: 19935879
-
Location, location, location: the role of cyclin D1 nuclear localization in cancer.J Cell Biochem. 2005 Dec 1;96(5):906-13. doi: 10.1002/jcb.20613. J Cell Biochem. 2005. PMID: 16163738 Review.
-
Control of metaphase-anaphase progression by proteolysis: cyclosome function regulated by the protein kinase A pathway, ubiquitination and localization.Philos Trans R Soc Lond B Biol Sci. 1999 Sep 29;354(1389):1559-69; discussion 1569-70. doi: 10.1098/rstb.1999.0499. Philos Trans R Soc Lond B Biol Sci. 1999. PMID: 10582241 Free PMC article. Review.
Cited by
-
Hepatic Ischemia-Reperfusion Impairs Blood-Brain Barrier Partly Due to Release of Arginase From Injured Liver.Front Pharmacol. 2021 Oct 13;12:724471. doi: 10.3389/fphar.2021.724471. eCollection 2021. Front Pharmacol. 2021. PMID: 34721021 Free PMC article.
-
Expression Of Cyclin D1 Protein Isoforms And Its Prognostic Significance In Cervical Cancer.Cancer Manag Res. 2019 Oct 24;11:9073-9083. doi: 10.2147/CMAR.S224026. eCollection 2019. Cancer Manag Res. 2019. PMID: 31695498 Free PMC article.
-
Copper Chaperone Atox1 Interacts with Cell Cycle Proteins.Comput Struct Biotechnol J. 2018 Oct 31;16:443-449. doi: 10.1016/j.csbj.2018.10.018. eCollection 2018. Comput Struct Biotechnol J. 2018. PMID: 30455854 Free PMC article.
-
Untangling the Role of MYC in Sarcomas and Its Potential as a Promising Therapeutic Target.Int J Mol Sci. 2025 Feb 25;26(5):1973. doi: 10.3390/ijms26051973. Int J Mol Sci. 2025. PMID: 40076599 Free PMC article. Review.
-
Function and regulation of transcription factors during mitosis-to-G1 transition.Open Biol. 2022 Jun;12(6):220062. doi: 10.1098/rsob.220062. Epub 2022 Jun 1. Open Biol. 2022. PMID: 35642493 Free PMC article. Review.
References
-
- Murray A. W. (2004) Recycling the cell cycle: cyclins revisited. Cell 116, 221–234 - PubMed
-
- Nevins J. R. (2001) The Rb/E2F pathway and cancer. Hum. Mol. Genet. 10, 699–703 - PubMed
-
- Dyson N. (1998) The regulation of E2F by pRB-family proteins. Genes Dev. 12, 2245–2262 - PubMed
-
- Massagué J. (2004) G1 cell-cycle control and cancer. Nature 432, 298–306 - PubMed
-
- Masamha C. P., and Benbrook D. M. (2009) Cyclin D1 degradation is sufficient to induce G1 cell cycle arrest despite constitutive expression of cyclin E2 in ovarian cancer cells. Cancer Res. 69, 6565–6572 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

