Early immune responses are independent of RGC dysfunction in glaucoma with complement component C3 being protective
- PMID: 28446616
- PMCID: PMC5441748
- DOI: 10.1073/pnas.1608769114
Early immune responses are independent of RGC dysfunction in glaucoma with complement component C3 being protective
Abstract
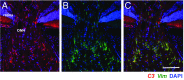
Various immune response pathways are altered during early, predegenerative stages of glaucoma; however, whether the early immune responses occur secondarily to or independently of neuronal dysfunction is unclear. To investigate this relationship, we used the Wlds allele, which protects from axon dysfunction. We demonstrate that DBA/2J.Wlds mice develop high intraocular pressure (IOP) but are protected from retinal ganglion cell (RGC) dysfunction and neuroglial changes that otherwise occur early in DBA/2J glaucoma. Despite this, immune pathways are still altered in DBA/2J.Wlds mice. This suggests that immune changes are not secondary to RGC dysfunction or altered neuroglial interactions, but may be directly induced by the increased strain imposed by high IOP. One early immune response following IOP elevation is up-regulation of complement C3 in astrocytes of DBA/2J and DBA/2J.Wlds mice. Unexpectedly, because the disruption of other complement components, such as C1Q, is protective in glaucoma, C3 deficiency significantly increased the number of DBA/2J eyes with nerve damage and RGC loss at an early time point after IOP elevation. Transcriptional profiling of C3-deficient cultured astrocytes implicated EGFR signaling as a hub in C3-dependent responses. Treatment with AG1478, an EGFR inhibitor, also significantly increased the number of DBA/2J eyes with glaucoma at the same early time point. These findings suggest that C3 protects from early glaucomatous damage, a process that may involve EGFR signaling and other immune responses in the optic nerve head. Therefore, therapies that target specific components of the complement cascade, rather than global inhibition, may be more applicable for treating human glaucoma.
Keywords: EGFR; WLDS; astrocytes; complement; glaucoma.
Conflict of interest statement
The authors declare no conflict of interest.
Figures








References
-
- Nickells RW, Howell GR, Soto I, John SW. Under pressure: Cellular and molecular responses during glaucoma, a common neurodegeneration with axonopathy. Annu Rev Neurosci. 2012;35:153–179. - PubMed
-
- Leske MC, Wu SY, Hennis A, Honkanen R, Nemesure B. BESs Study Group Risk factors for incident open-angle glaucoma: The Barbados Eye Studies. Ophthalmology. 2008;115:85–93. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

