Dense Array of Spikes on HIV-1 Virion Particles
- PMID: 28446665
- PMCID: PMC5487557
- DOI: 10.1128/JVI.00415-17
Dense Array of Spikes on HIV-1 Virion Particles
Abstract
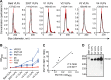
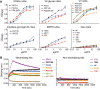
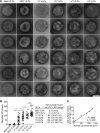
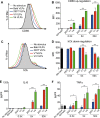
HIV-1 is rare among viruses for having a low number of envelope glycoprotein (Env) spikes per virion, i.e., ∼7 to 14. This exceptional feature has been associated with avoidance of humoral immunity, i.e., B cell activation and antibody neutralization. Virus-like particles (VLPs) with increased density of Env are being pursued for vaccine development; however, these typically require protein engineering that alters Env structure. Here, we used instead a strategy that targets the producer cell. We employed fluorescence-activated cell sorting (FACS) to sort for cells that are recognized by trimer cross-reactive broadly neutralizing antibody (bnAb) and not by nonneutralizing antibodies. Following multiple iterations of FACS, cells and progeny virions were shown to display higher levels of antigenically correct Env in a manner that correlated between cells and cognate virions (P = 0.027). High-Env VLPs, or hVLPs, were shown to be monodisperse and to display more than a 10-fold increase in spikes per particle by electron microscopy (average, 127 spikes; range, 90 to 214 spikes). Sequencing revealed a partial truncation in the C-terminal tail of Env that had emerged in the sort; however, iterative rounds of "cell factory" selection were required for the high-Env phenotype. hVLPs showed greater infectivity than standard pseudovirions but largely similar neutralization sensitivity. Importantly, hVLPs also showed superior activation of Env-specific B cells. Hence, high-Env HIV-1 virions, obtained through selection of producer cells, represent an adaptable platform for vaccine design and should aid in the study of native Env.IMPORTANCE The paucity of spikes on HIV is a unique feature that has been associated with evasion of the immune system, while increasing spike density has been a goal of vaccine design. Increasing the density of Env by modifying it in various ways has met with limited success. Here, we focused instead on the producer cell. Cells that stably express HIV spikes were screened on the basis of high binding by bnAbs and low binding by nonneutralizing antibodies. Levels of spikes on cells correlated well with those on progeny virions. Importantly, high-Env virus-like particles (hVLPs) were produced with a manifest array of well-defined spikes, and these were shown to be superior in activating desirable B cells. Our study describes HIV particles that are densely coated with functional spikes, which should facilitate the study of HIV spikes and their development as immunogens.
Keywords: HIV-1; antigenicity; broadly neutralizing antibodies; cellPACK; electron microscopy; envelope glycoprotein; fluorescence-activated cell sorting; vaccine design; viral infectivity; virus-like particles.
Copyright © 2017 American Society for Microbiology.
Figures






References
-
- Chertova E, Bess JW Jr, Crise BJ, Sowder IR, Schaden TM, Hilburn JM, Hoxie JA, Benveniste RE, Lifson JD, Henderson LE, Arthur LO. 2002. Envelope glycoprotein incorporation, not shedding of surface envelope glycoprotein (gp120/SU), is the primary determinant of SU content of purified human immunodeficiency virus type 1 and simian immunodeficiency virus. J Virol 76:5315–5325. doi: 10.1128/JVI.76.11.5315-5325.2002. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

