Antimalarial efficacy of MMV390048, an inhibitor of Plasmodium phosphatidylinositol 4-kinase
- PMID: 28446690
- PMCID: PMC5731459
- DOI: 10.1126/scitranslmed.aad9735
Antimalarial efficacy of MMV390048, an inhibitor of Plasmodium phosphatidylinositol 4-kinase
Abstract
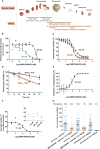
As part of the global effort toward malaria eradication, phenotypic whole-cell screening revealed the 2-aminopyridine class of small molecules as a good starting point to develop new antimalarial drugs. Stemming from this series, we found that the derivative, MMV390048, lacked cross-resistance with current drugs used to treat malaria. This compound was efficacious against all Plasmodium life cycle stages, apart from late hypnozoites in the liver. Efficacy was shown in the humanized Plasmodium falciparum mouse model, and modest reductions in mouse-to-mouse transmission were achieved in the Plasmodium berghei mouse model. Experiments in monkeys revealed the ability of MMV390048 to be used for full chemoprotection. Although MMV390048 was not able to eliminate liver hypnozoites, it delayed relapse in a Plasmodium cynomolgi monkey model. Both genomic and chemoproteomic studies identified a kinase of the Plasmodium parasite, phosphatidylinositol 4-kinase, as the molecular target of MMV390048. The ability of MMV390048 to block all life cycle stages of the malaria parasite suggests that this compound should be further developed and may contribute to malaria control and eradication as part of a single-dose combination treatment.
Copyright © 2017, American Association for the Advancement of Science.
Conflict of interest statement
Figures



References
-
- World Health Organization. World Malaria Report 2015. World Health Organization; 2015.
-
- Mbengue A, Bhattacharjee S, Pandharkar T, Liu H, Estiu G, Stahelin RV, Rizk SS, Njimoh DL, Ryan Y, Chotivanich K, Nguon C, Ghorbal M, Lopez-Rubio JJ, Pfrender M, Emrich S, Mohandas N, Dondorp AM, Wiest O, Haldar K. A molecular mechanism of artemisinin resistance in Plasmodium falciparum malaria. Nature. 2015;520:683–687. - PMC - PubMed
-
- Fidock DA. Drug discovery: Priming the antimalarial pipeline. Nature. 2010;465:297–298. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

