Mammalian Target of Rapamycin (mTOR) Regulates Transforming Growth Factor-β1 (TGF-β1)-Induced Epithelial-Mesenchymal Transition via Decreased Pyruvate Kinase M2 (PKM2) Expression in Cervical Cancer Cells
- PMID: 28446743
- PMCID: PMC5417590
- DOI: 10.12659/msm.901542
Mammalian Target of Rapamycin (mTOR) Regulates Transforming Growth Factor-β1 (TGF-β1)-Induced Epithelial-Mesenchymal Transition via Decreased Pyruvate Kinase M2 (PKM2) Expression in Cervical Cancer Cells
Abstract
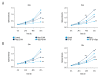
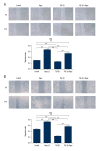
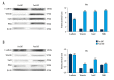
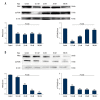
BACKGROUND Epithelial-mesenchymal transition (EMT) plays an important role in cancer tumorigenesis. Transforming growth factor β1 (TGF-β1) can induced EMT, which could increase tumor migration and invasion. Moreover, recent studies have been proven that mammalian target of rapamycin (mTOR) is a critical regulator of EMT. We investigated the mechanisms of mTOR in transforming growth factor β1 (TGF-β1)-induced EMT in cervical cancer cells. MATERIAL AND METHODS HeLa and SiHa cells were treated with 10 ng/ml TGF-β1 to induce EMT. Then, they were treated with or without rapamycin. CCK8 assay was performed to determine cell proliferation. Cell migration was detected by wound-healing assay; apoptosis was analyzed by flow cytometry; mTOR inhibitors inhibited mTOR pathway to assess the expression of E-cadherin, Vimentin STAT3, Snail2, p-p70s6k, and PKM2 expression. RESULTS TGF-β1 promoted proliferation and migration, and attenuated apoptosis in cervical carcinoma cells. Rapamycin abolished TGF-β1-induced EMT cell proliferation and migration and reversed TGF-β1-induced EMT. E-cadherin were suppressed, whereas Vimentin and PKM2 were increased in HeLa and SiHa cells after stimulation with TGF-β1. Moreover, mTOR was activated in the process of TGF-β1-induced EMT. Rapamycin inhibited the phosphorylation of p70s6k. Furthermore, inhibition of the mTOR pathway decreased PKM2 expression. CONCLUSIONS Inhibition of the mTOR pathway abolished TGF-β1-induced EMT and reduced mTOR/p70s6k signaling, which downregulated PKM2 expression. Our results provide novel mechanistic insight into the anti-tumor effects of inhibition of mTOR.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





References
-
- Maeda M, Johnson KR, Wheelock MJ. Cadherin switching: Essential for behavioral but not morphological changes during an epithelium-to-mesenchyme transition. J Cell Sci. 2005;118(5):873–87. - PubMed
-
- Voulgari A, Pintzas A. Epithelial-mesenchymal transition in cancer metastasis: mechanisms, markers and strategies to overcome drug resistance in the clinic. Biochim Biophys Acta. 2009;1796(2):75–90. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

