Therapeutic effects of anti-HMGB1 monoclonal antibody on pilocarpine-induced status epilepticus in mice
- PMID: 28446773
- PMCID: PMC5430706
- DOI: 10.1038/s41598-017-01325-y
Therapeutic effects of anti-HMGB1 monoclonal antibody on pilocarpine-induced status epilepticus in mice
Abstract
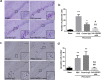
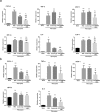
Inflammatory processes in brain tissue have been described in human epilepsy of various etiologies and in experimental models of seizures. High mobility group box-1 (HMGB1) is now recognized as representative of damage-associated molecular patterns (DAMPs). In the present study, we focused on whether anti-HMGB1 antibody treatment could relieve status epilepticus- triggered BBB breakdown and inflammation response in addition to the seizure behavior itself. Pilocarpine and methyl-scopolamine were used to establish the acute seizure model. Anti-HMGB1 mAb showed inhibitory effects on leakage of the BBB, and on the HMGB1 translocation induced by pilocarpine. The expression of inflammation-related factors, such as MCP-1, CXCL-1, TLR-4, and IL-6 in hippocampus and cerebral cortex were down-regulated by anti-HMGB1 mAb associated with the number of activated astrocytes, microglial cells as well as the expression of IL-1β. Both hematoxylin & eosin and TUNEL staining showed that the apoptotic cells could be reduced after anti-HMGB1 mAb treatment. The onset and latency of Racine stage five were significantly prolonged in the anti-HMGB1 mAb group. These results suggested that anti-HMGB1 mAb prevented the BBB permeability, reduced HMGB1 translocation while inhibiting the expression of inflammation-related factors, protected against neural cell apoptosis and prolonged Racine stage 5 seizure onset and latency.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures







References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

