In Vitro and In Vivo Profile of PPL-101 and PPL-103: Mixed Opioid Partial Agonist Analgesics with Low Abuse Potential
- PMID: 28446883
- PMCID: PMC5388777
- DOI: 10.3389/fpsyt.2017.00052
In Vitro and In Vivo Profile of PPL-101 and PPL-103: Mixed Opioid Partial Agonist Analgesics with Low Abuse Potential
Abstract
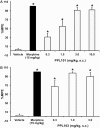
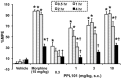
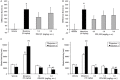
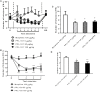
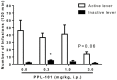
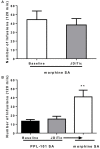
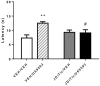
Opiates are still the most effective and widely used treatments for acute and chronic pain. However, the problems associated with morphine and other standard opioid analgesics severely limit their effectiveness in the clinic. PPL-101 and PPL-103 derived from morphine and morphinan ring systems contain a chiral N-substituent, which confers it with a unique combination of high-binding affinities and partial agonist activities at mu, delta, and kappa opioid receptors, leading to unique in vivo pharmacology compared to other conventional opioids. Acute antinociceptive and reward acquisition of PPL-101 and PPL-103 were assessed in mice using the tail flick assay and conditioned place preference (CPP) paradigm, respectively. The reinforcing effects of these compounds were assessed in rats using the self-administration paradigm. In mice, PPL-101 and PPL-103 produced antinociception reaching maximal effects that were equivalent to morphine at approximately 1/3 and 1/10 of morphine's dose, respectively. PPL-101-induced antinociception was attenuated following pretreatment with the kappa antagonist JDTic, but not the mu opioid antagonist beta-FNA. In mice, PPL-101 and PPL-103 produced dose-dependent decreases in activity, similar to other kappa agonists; however, they did not produce conditioned place aversion, and in fact elicited a trend toward CPP. In rats, neither PPL-101 nor PPL-103 were self-administered when substituted for morphine and PPL-101 attenuated morphine self-administration, when administered systemically prior to the self-administration session. Collectively, these results indicate that mixed opioid receptor partial agonists can produce potent antinociceptive activity with a lack of aversion in mice and without being self-administered in rats. Compounds with this profile could be superior analgesics with greatly reduced addiction liability and fewer side-effects compared to traditional opiates.
Keywords: analgesic; conditioned place preference; kappa opiate; non-addicting; self-administration.
Figures

References
-
- Porreca F, Mosberg HI, Hurst R, Hruby VJ, Burks TF. Roles of mu, delta and kappa opioid receptors in spinal and supraspinal mediation of gastrointestinal transit effects and hot-plate analgesia in the mouse. J Pharmacol Exp Ther (1984) 230:341–8. - PubMed
-
- Howell LL, Bergman J, Morse WH. Effects of levorphanol and several kappa-selective opioids on respiration and behavior in rhesus monkeys. J Pharmacol Exp Ther (1988) 245:364–72. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

