Regulation and Molecular Basis of Environmental Muropeptide Uptake and Utilization in Fastidious Oral Anaerobe Tannerella forsythia
- PMID: 28446907
- PMCID: PMC5388701
- DOI: 10.3389/fmicb.2017.00648
Regulation and Molecular Basis of Environmental Muropeptide Uptake and Utilization in Fastidious Oral Anaerobe Tannerella forsythia
Abstract
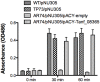
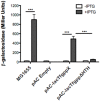
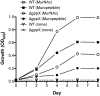
Tannerella forsythia is a Gram-negative oral anaerobe associated with periodontitis. This bacterium is auxotrophic for the peptidoglycan amino sugar N-acetylmuramic (MurNAc) and likely relies on scavenging peptidoglycan fragments (muropeptides) released by cohabiting bacteria during their cell wall recycling. Many Gram-negative bacteria utilize an inner membrane permease, AmpG, to transport peptidoglycan fragments into their cytoplasm. In the T. forsythia genome, the Tanf_08365 ORF has been identified as a homolog of AmpG permease. In order to confirm the functionality of Tanf_08365, a reporter system in an Escherichia coli host was generated that could detect AmpG-dependent accumulation of cytosolic muropeptides via a muropeptide-inducible β-lactamase reporter gene. In trans complementation of this reporter strain with a Tanf_08365 containing plasmid caused significant induction of β-lactamase activity compared to that with an empty plasmid control. These data indicated that Tanf_08365 acted as a functional muropeptide permease causing accumulation of muropeptides in E. coli and thus suggested that it is a permease involved in muropeptide scavenging in T. forsythia. Furthermore, we showed that the promoter regulating the expression of Tanf_08365 was activated significantly by a hybrid two-component system regulatory protein, GppX. We also showed that compared to the parental T. forsythia strain a mutant lacking GppX in which the expression of AmpG was reduced significantly attenuated in utilizing free muropeptides. In summary, we have uncovered the mechanism by which this nutritionally fastidious microbe accesses released muropeptides in its environment, opening up the possibility of targeting this activity to reduce its numbers in periodontitis patients with potential benefits in the treatment of disease.
Keywords: AmpG; GppX; Tannerella forsythia; muropeptides; peptidoglycan.
Figures


Similar articles
-
N-Acetylmuramic Acid (MurNAc) Auxotrophy of the Oral Pathogen Tannerella forsythia: Characterization of a MurNAc Kinase and Analysis of Its Role in Cell Wall Metabolism.Front Microbiol. 2018 Jan 26;9:19. doi: 10.3389/fmicb.2018.00019. eCollection 2018. Front Microbiol. 2018. PMID: 29434575 Free PMC article.
-
Utilization of different MurNAc sources by the oral pathogen Tannerella forsythia and role of the inner membrane transporter AmpG.BMC Microbiol. 2020 Nov 17;20(1):352. doi: 10.1186/s12866-020-02006-z. BMC Microbiol. 2020. PMID: 33203363 Free PMC article.
-
Identification of a Novel N-Acetylmuramic Acid Transporter in Tannerella forsythia.J Bacteriol. 2016 Oct 21;198(22):3119-3125. doi: 10.1128/JB.00473-16. Print 2016 Nov 15. J Bacteriol. 2016. PMID: 27601356 Free PMC article.
-
Peptidoglycan synthesis in Tannerella forsythia: Scavenging is the modus operandi.Mol Oral Microbiol. 2018 Apr;33(2):125-132. doi: 10.1111/omi.12210. Epub 2018 Feb 12. Mol Oral Microbiol. 2018. PMID: 29247483 Free PMC article. Review.
-
Peptidoglycan Salvage Enables the Periodontal Pathogen Tannerella forsythia to Survive within the Oral Microbial Community.Microb Physiol. 2021;31(2):123-134. doi: 10.1159/000516751. Epub 2021 Jun 9. Microb Physiol. 2021. PMID: 34107471 Review.
Cited by
-
N-Acetylmuramic Acid (MurNAc) Auxotrophy of the Oral Pathogen Tannerella forsythia: Characterization of a MurNAc Kinase and Analysis of Its Role in Cell Wall Metabolism.Front Microbiol. 2018 Jan 26;9:19. doi: 10.3389/fmicb.2018.00019. eCollection 2018. Front Microbiol. 2018. PMID: 29434575 Free PMC article.
-
Investigating Peptidoglycan Recycling Pathways in Tannerella forsythia with N-Acetylmuramic Acid Bioorthogonal Probes.ACS Infect Dis. 2022 Sep 9;8(9):1831-1838. doi: 10.1021/acsinfecdis.2c00333. Epub 2022 Aug 4. ACS Infect Dis. 2022. PMID: 35924866 Free PMC article.
-
Tannerella forsythia scavenges Fusobacterium nucleatum secreted NOD2 stimulatory molecules to dampen oral epithelial cell inflammatory response.Mol Oral Microbiol. 2024 Apr;39(2):40-46. doi: 10.1111/omi.12429. Epub 2023 Jul 17. Mol Oral Microbiol. 2024. PMID: 37459655 Free PMC article.
-
NamZ1 and NamZ2 from the Oral Pathogen Tannerella forsythia Are Peptidoglycan Processing Exo-β-N-Acetylmuramidases with Distinct Substrate Specificities.J Bacteriol. 2022 Mar 15;204(3):e0059721. doi: 10.1128/jb.00597-21. Epub 2022 Feb 7. J Bacteriol. 2022. PMID: 35129368 Free PMC article.
-
Persistence of Tannerella forsythia and Fusobacterium nucleatum in dental plaque: a strategic alliance.Curr Oral Health Rep. 2020 Mar;7(1):22-28. doi: 10.1007/s40496-020-00254-6. Epub 2020 Jan 29. Curr Oral Health Rep. 2020. PMID: 36779221 Free PMC article.
References
-
- Graham M. R., Smoot L. M., Migliaccio C. A., Virtaneva K., Sturdevant D. E., Porcella S. F., et al. (2002). Virulence control in group A Streptococcus by a two-component gene regulatory system: global expression profiling and in vivo infection modeling. Proc. Natl. Acad. Sci. U.S.A. 99 13855–13860. 10.1073/pnas.202353699 - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

