A randomized trial of the effects of the no-carrageenan diet on ulcerative colitis disease activity
- PMID: 28447072
- PMCID: PMC5389019
- DOI: 10.3233/NHA-170023
A randomized trial of the effects of the no-carrageenan diet on ulcerative colitis disease activity
Abstract
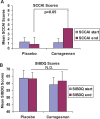
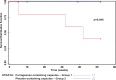
BACKGROUND: Carrageenan is a very common food additive in Western diets, but predictably causes inflammation in thousands of cell-based and animal experiments. OBJECTIVE: To assess the impact of carrageenan exposure on the interval to relapse in patients with ulcerative colitis in remission. METHODS: A randomized, double-blind, placebo-controlled, multicenter, clinical trial was conducted to assess if patients with ulcerative colitis in remission would have a longer interval to relapse if they followed a diet with no carrageenan. All participants were instructed in the no-carrageenan diet and were randomized to either placebo capsules or carrageenan-containing capsules. The carrageenan in the capsules was less than the average daily carrageenan intake from the diet. Relapse was defined as an increase of two or more points on the Simple Clinical Colitis Activity Index (SCCAI) and intensification of treatment for ulcerative colitis. Participants were followed by telephone calls every two weeks until relapse or one year of participation. The occurrence of relapse and inflammatory biomarkers were compared between the two groups. RESULTS: Twelve patients completed study questionnaires. Three patients who received carrageenan-containing capsules relapsed, and none of the patients who received placebo-containing capsules relapsed (p = 0.046, log-rank test). Laboratory tests showed increases in Interleukin-6 (p = 0.02, paired t-test, two-tailed) and fecal calprotectin (p = 0.06; paired t-test, two-tailed) between the beginning and the end of study participation in the carrageenan-exposed group, but not in the placebo-group. CONCLUSION: Carrageenan intake contributed to earlier relapse in patients with ulcerative colitis in remission. Restriction of dietary carrageenan may benefit patients with ulcerative colitis.
Keywords: Colitis; Interleukin-6; carrageenan; food additive; inflammation.
Figures
Comment in
-
Reply to critique of "A randomized trial of the effects of the no-carrageenan diet on ulcerative colitis disease activity".Nutr Healthy Aging. 2019;5(2):159-163. doi: 10.3233/NHA-190068. Epub 2019 Sep 23. Nutr Healthy Aging. 2019. PMID: 31922053 Free PMC article.
References
-
- IARC Working Group on the Evaluation of the Carcinogenic Risk of Chemicals to Humans, Carrageenan. IARC monogr. Eval Carcino Risk Hum Lyon. 1983;31:79–94. - PubMed
-
- Blakemore WR, Harrell AR. Carrageenan In: Meson A, Ed. Food Stabilizers, Thickeners, and Gelling Agents, Chapter 5 Hoboken: Wiley-Blackwell, Blackwell Publishing Ltd, 2011, pp. 73–94.
-
- Campo VL, Kawano DF, da Silva DB Jr, Carvalho I. Carrageenans: Biological properties, chemical modifications and structural analysis –A review. Carbohydr Polym. 2009;77(2):167–80.
-
- Liu J, Zhan X, Wan J, Wang Y, Wang C. Review for carrageenan-based pharmaceutical biomaterials. Favourable physical features versus adverse biological effects. Carbohydr Polym. 2015;121:27–36. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
