Pathogenic role and therapeutic potential of pleiotrophin in mouse models of ocular vascular disease
- PMID: 28447229
- PMCID: PMC5658272
- DOI: 10.1007/s10456-017-9557-6
Pathogenic role and therapeutic potential of pleiotrophin in mouse models of ocular vascular disease
Abstract
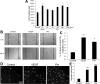
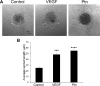
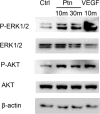
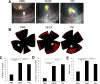
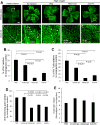
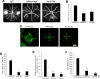
Angiogenic factors play an important role in the pathogenesis of diabetic retinopathy (DR), neovascular age-related macular degeneration (nAMD) and retinopathy of prematurity (ROP). Pleiotrophin, a well-known angiogenic factor, was recently reported to be upregulated in the vitreous fluid of patients with proliferative DR (PDR). However, its pathogenic role and therapeutic potential in ocular vascular diseases have not been defined in vivo. Here using corneal pocket assays, we demonstrated that pleiotrophin induced angiogenesis in vivo. To investigate the pathological role of pleiotrophin we used neutralizing antibody to block its function in multiple in vivo models of ocular vascular diseases. In a mouse model of DR, intravitreal injection of pleiotrophin-neutralizing antibody alleviated diabetic retinal vascular leakage. In a mouse model of oxygen-induced retinopathy (OIR), which is a surrogate model of ROP and PDR, we demonstrated that intravitreal injection of anti-pleiotrophin antibody prevented OIR-induced pathological retinal neovascularization and aberrant vessel tufts. Finally, pleiotrophin-neutralizing antibody ameliorated laser-induced choroidal neovascularization, a mouse model of nAMD, suggesting that pleiotrophin is involved in choroidal vascular disease. These findings suggest that pleiotrophin plays an important role in the pathogenesis of DR with retinal vascular leakage, ROP with retinal neovascularization and nAMD with choroidal neovascularization. The results also support pleiotrophin as a promising target for anti-angiogenic therapy.
Keywords: Anti-angiogenic therapy; Choroidal neovascularization; Diabetic retinopathy; Neovascular age-related macular degeneration; Oxygen-induced retinopathy; Pleiotrophin; Retinopathy of prematurity.
Conflict of interest statement
Figures
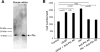
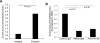
References
-
- Wong WL, Su X, Li X, Cheung CM, Klein R, Cheng CY, et al. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob Health. 2014;2(2):e106–16. - PubMed
-
- Votruba M, Gregor Z. Neovascular age-related macular degeneration: present and future treatment options. Eye (Lond) 2001;15(Pt 3):424–9. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

