Regulation of Cardiomyocyte T-Tubular Structure: Opportunities for Therapy
- PMID: 28447290
- PMCID: PMC5423965
- DOI: 10.1007/s11897-017-0329-9
Regulation of Cardiomyocyte T-Tubular Structure: Opportunities for Therapy
Abstract
Purpose of review: Membrane invaginations called t-tubules play an integral role in triggering cardiomyocyte contraction, and their disruption during diseases such as heart failure critically impairs cardiac performance. In this review, we outline the growing understanding of the malleability of t-tubule structure and function, and highlight emerging t-tubule regulators which may be exploited for novel therapies.
Recent findings: New technologies are revealing the nanometer scale organization of t-tubules, and their functional junctions with the sarcoplasmic reticulum called dyads, which generate Ca2+ sparks. Recent data have indicated that the dyadic anchoring protein junctophilin-2, and the membrane-bending protein BIN1 are key regulators of dyadic formation and maintenance. While the underlying signals which control expression and localization of these proteins remain unclear, accumulating data support an important role of myocardial workload. Although t-tubule alterations are believed to be a key cause of heart failure, the plasticity of these structures also creates an opportunity for therapy. Promising recent data suggest that such therapies may specifically target junctophilin-2, BIN1, and/or mechanotransduction.
Keywords: Bridging integrator-1; Calcium homeostasis; Cardiomyocytes; Heart failure; Junctophilin-2; T-tubules.
Conflict of interest statement
Conflict of Interest
Ornella Manfra, Michael Frisk, and William E. Louch declare no conflict of interest.
Human and Animal Rights
Previously unpublished images of t-tubules in human ventricular tissue (Fig. 1a, left panels) were performed on biopsies taken from healthy organ donors with hearts deemed unsuitable for transplant. This work was approved by the Regional Ethics Committee (project S-05172) in agreement with The Declaration of Helsinki and the Council of Europe Convention on Human Rights and Biomedicine.
Funding
Generous funding has been provided by the European Union’s Horizon 2020 research and innovation program (Consolidator grant, WEL) under grant agreement No 647714. Additional support has been received from The South-Eastern Norway Regional Health Authority, Anders Jahre’s Fund for the Promotion of Science, The Norwegian Institute of Public Health, Oslo University Hospital Ullevål, and the University of Oslo.
Figures
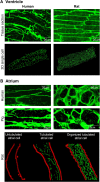

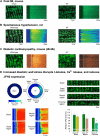
References
-
- Lindner E. Submicroscopic morphology of the cardiac muscle. Zeitschrift fur Zellforschung und mikroskopische Anatomie (Vienna, Austria: 1948) 1957;45(6):702–746. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

