Chemotherapy alone versus chemotherapy plus radiotherapy for adults with early stage Hodgkin lymphoma
- PMID: 28447341
- PMCID: PMC6478261
- DOI: 10.1002/14651858.CD007110.pub3
Chemotherapy alone versus chemotherapy plus radiotherapy for adults with early stage Hodgkin lymphoma
Update in
-
Chemotherapy alone versus chemotherapy plus radiotherapy for adults with early-stage Hodgkin's lymphoma.Cochrane Database Syst Rev. 2024 Dec 2;12(12):CD007110. doi: 10.1002/14651858.CD007110.pub4. Cochrane Database Syst Rev. 2024. PMID: 39620432
Abstract
Background: Combined modality treatment consisting of chemotherapy followed by localised radiotherapy is the standard treatment for patients with early stage Hodgkin lymphoma (HL). However, due to long- term adverse effects such as secondary malignancies the role of radiotherapy has been questioned recently and some clinical study groups advocate chemotherapy only for this indication.
Objectives: To assess the effects of chemotherapy alone compared to chemotherapy plus radiotherapy in adults with early stage HL .
Search methods: For the or i ginal version of this review, we searched MEDLINE, Embase and CENTRAL as well as conference proceedings (American Society of Hematology, American Society of Clinical Oncology and International Symposium of Hodgkin Lymphoma) from January 1980 to November 2010 for randomised controlled trials (RCTs) comparing chemotherapy alone versus chemotherapy regimens plus radiotherapy. For the updated review we searched MEDLINE, CENTRAL and conference proceedings to December 2016.
Selection criteria: We included RCTs comparing chemotherapy alone with chemotherapy plus radiotherapy in patients with early stage HL. We excluded trials with more than 20% of patients in advanced stage. As the value of radiotherapy in addition to chemotherapy is still not clear, we also compared to more cycles of chemotherapy in the control arm. In this updated review, we also included a second comparison evaluating trials with varying numbers of cycles of chemotherapy between intervention and control arms, same chemotherapy regimen in both arms assumed. We excluded trials evaluating children only, therefore only trials involving adults are included in this updated review.
Data collection and analysis: Two review authors independently extracted data and assessed the quality of trials. We contacted study authors to obtain missing information. As effect measures we used hazard ratios (HR) for overall survival (OS) and progression-free survival (PFS) and risk ratios (RR) for response rates. Since not all trials reported PFS according to our definitions, we evaluated all similar outcomes (e.g. event-free survival) as PFS/tumour control.
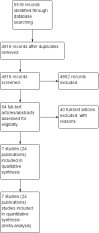
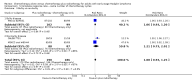
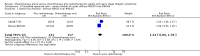
Main results: Our search led to 5518 potentially relevant references. From these, we included seven RCTs in the analyses involving 2564 patients. In contrast to the first version of this review including five trials, we excluded trials randomising children. As a result, we excluded one trial from the former analyses and we identified three new trials.Five trials with 1388 patients compared the combination of chemotherapy alone and chemotherapy plus radiotherapy, with the same number of chemotherapy cycles in both arms. The addition of radiotherapy to chemotherapy has probably little or no difference on OS (HR 0.48; 95% confidence interval (CI) 0.22 to 1.06; P = 0.07, moderate- quality evidence), however two included trials had potential other high risk of bias due to a high number of patients not receiving planned radiotherapy. After excluding these trials in a sensitivity analysis, the results showed that the combination of chemotherapy and radiotherapy improved OS compared to chemotherapy alone (HR 0.31; 95% CI 0.19 to 0.52; P <0.00001, moderate- quality evidence). In contrast to chemotherapy alone the use of chemotherapy and radiotherapy improved PFS (HR 0.42; 95% CI 0.25 to 0.72; P = 0.001; moderate- quality evidence). Regarding infection- related mortality (RR 0.33; 95% CI 0.01 to 8.06; P = 0.5; low- quality evidence), second cancer- related mortality (RR 0.53; 95% CI 0.07 to 4.29; P = 0.55; low- quality evidence) and cardiac disease- related mortality (RR 2.94; 95% CI 0.31 to 27.55; P = 0.35;low- quality evidence), there is no evidence for a difference between the use of chemotherapy alone and chemotherapy plus radiotherapy. For complete response rate (CRR) (RR 1.08; 95% CI 0.93 to 1.25; P = 0.33; low- quality evidence), there is also no evidence for a difference between treatment groups.Two trials with 1176 patients compared the combination of chemotherapy alone and chemotherapy plus radiotherapy, with different numbers of chemotherapy cycles in both arms. OS is reported in one trial only, the use of chemotherapy alone (more chemotherapy cycles) may improve OS compared to chemotherapy plus radiotherapy (HR 2.12; 95% CI 1.03 to 4.37; P = 0.04; low- quality evidence). This trial also had a potential other high risk of bias due to a high number of patients not receiving planned therapy. There is no evidence for a difference between chemotherapy alone and chemotherapy plus radiotherapy regarding PFS (HR 0.42; 95% CI 0.14 to 1.24; P = 0.12; low- quality evidence). After excluding the trial with patients not receiving the planned therapy in a sensitivity analysis, the results showed that the combination of chemotherapy and radiotherapy improved PFS compared to chemotherapy alone (HR 0.24; 95% CI 0.070 to 0.88; P = 0.03, based on one trial). For infection- related mortality (RR 6.90; 95% CI 0.36 to 132.34; P = 0.2; low- quality evidence), second cancer- related mortality (RR 2.22; 95% CI 0.7 to 7.03; P = 0.18; low- quality evidence) and cardiac disease-related mortality (RR 0.99; 95% CI 0.14 to 6.90; P = 0.99; low-quality evidence), there is no evidence for a difference between the use of chemotherapy alone and chemotherapy plus radiotherapy. CRR rate was not reported.
Authors' conclusions: This systematic review compared the effects of chemotherapy alone and chemotherapy plus radiotherapy in adults with early stage HL .For the comparison with same numbers of chemotherapy cycles in both arms, we found moderate- quality evidence that PFS is superior in patients receiving chemotherapy plus radiotherapy than in those receiving chemotherapy alone. The addition of radiotherapy to chemotherapy has probably little or no difference on OS . The sensitivity analysis without the trials with potential other high risk of bias showed that chemotherapy plus radiotherapy improves OS compared to chemotherapy alone.For the comparison with different numbers of chemotherapy cycles between the arms there are no implications for OS and PFS possible, because of the low quality of evidence of the results.
Conflict of interest statement
Blank O: no known conflict of interest.
Monsef I: no known conflict of interest.
Specht L: no known conflict of interest.
Engert A: no known conflict of interest.
Skoetz N: no known conflict of interest.
von Tresckow B: no known conflict of interest.
Figures

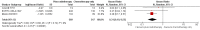
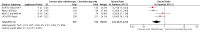
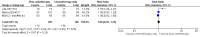
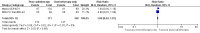




























































Update of
-
Chemotherapy alone versus chemotherapy plus radiotherapy for early stage Hodgkin lymphoma.Cochrane Database Syst Rev. 2011 Feb 16;(2):CD007110. doi: 10.1002/14651858.CD007110.pub2. Cochrane Database Syst Rev. 2011. Update in: Cochrane Database Syst Rev. 2017 Apr 27;4:CD007110. doi: 10.1002/14651858.CD007110.pub3. PMID: 21328291 Updated.
References
References to studies included in this review
CALGB 7751 {published data only}
-
- Bloomfield CD, Pajak TF, Glicksman AS, Gottlieb AJ, Coleman M, Nissen NI, et al. Chemotherapy and combined modality therapy for Hodgkin's disease: a progress report on Cancer and Leukemia Group B studies. Cancer Treatment Reports 1982; Vol. 66, issue 4:835‐46. - PubMed
EORTC‐GELA H9‐F {published data only}
-
- Eghbali H, Brice P, Creemers GY, Kooji MM, Carde P, Van't Veer MB, et al. Comparson of three radiation dose levels after EBVP regimen in favourable supradiaphragmatic clinical stages (CS) I‐II Hodgkin's lymphoma (HL): Preliminary results of EORTC‐GELA H9‐F trial. Blood 2005;106(11 suppl):abstract 814.
-
- Noordijk EM, Thomas J, Ferme C, 't Veer MB, Brice P, Divine M, et al. First results of EORTC‐GELA H9 randomized trials: the H9‐F trial (comparing 3 radiation dose levels) and H9‐U trial (comparing 3 chemotherapy schemes) in patients with favourable or unfavourable early stage Hodgkin's lymphoma (HL). Journal of Clinical Oncology 2005; Vol. 23, issue suppl:abstract 6505.
-
- Noordijk EM, Thomas J, Fermé C, Van't Veer MB, Brice P, Divine M, et al. First results of the EORTC‐GELA H9 randomized trials: the H9‐F trial (comparing 3 radiation dose levels) and H9‐U trial (comparing 3 chemotherapy schemes) in patients with favorable or unfavorable early stage Hodgkin's lymphoma (HL). ASCO Annual Meeting Presentation Slides YR:2005.
-
- Thomas J, Ferme C, Noordijk EM, Rieux C, Hennequin C, Lybeert MLM, et al. Six cycles of EBVP followed by 36 Gy involvedfield irradiation vs. no irradiation in favourable supradiaphragmatic clinical stages III Hodgkin's lymphoma: the EORTCGELA strategy in 771 patients (H9‐F trial‐20982) [Abstract AE11a]. European Journal of Haematology 2004;73(Supp. 65):40.
-
- Thomas J, Fermé C, Noordijk EM, Eghbali H, Henry‐Amar M. The EORTC‐GELA treatment strategy in clinical stages I‐II HL: Results of the H9‐F and H9‐U trials. International Symposium on Hodgkin Lymphoma, Cologne, Presentation YR:2007.
H10F {published data only}
-
- Andre MPE. An update on the EORTC / LYSA / FIL H10 trial. 9th International Symposium on Hodgkin Lymphoma Cologne, Germany 2013.
-
- Andre MPE, Reman O, Federico M, Girinski T, Brice P, Brusamolino E, et al. Interim analysis of the randomized EORTC/LYSA/FIl Intergroup H10 trial on early PET‐scan driven treatment adaptation in stage I/II Hodgkin lymphoma. Blood 2012;120:549.
-
- Raemaekers JM, André MP, Federico M, Girinsky T, Oumedaly R, Brusamolino E, et al. Omitting radiotherapy in early positron emission tomography‐negative stage I/II Hodgkin lymphoma is associated with an increased risk of early relapse: Clinical results of the preplanned interim analysis of the randomized EORTC/LYSA/FIL H10 trial. Journal of Clinical Oncology 2014;32(12):1188‐94. - PubMed
H10U {published data only}
-
- * Raemaekers JM, André MP, Federico M, Girinsky T, Oumedaly R, Brusamolino E, et al. Omitting radiotherapy in early positron emission tomography‐negative stage I/II Hodgkin lymphoma is associated with an increased risk of early relapse: Clinical results of the preplanned interim analysis of the randomized EORTC/LYSA/FIL H10 trial. Journal of Clinical Oncology 2014;32(12):1188‐94. - PubMed
-
- Andre MPE. An update on the EORTC / LYSA / FIL H10 trial. 9th International Symposium on Hodgkin Lymphoma Cologne, Germany 2013.
-
- Andre MPE, Reman O, Federico M, Girinski T, Brice P, Brusamolino E, et al. Interim analysis of the randomized EORTC/LYSA/FIl Intergroup H10 trial on early PET‐scan driven treatment adaptation in stage I/II Hodgkin lymphoma. Blood 2012;120:549.
HD6 {published data only}
-
- Meyer R, Gospodarowicz M, Connors J, Pearcey R, Bezjak A, Wells W. A randomized phase III comparison of single‐modality ABVD with a strategy that includes radiation therapy in patients with early‐stage Hodgkin's Disease: the HD‐6 trial of the National Cancer Institute of Canada Clinical Trials Group (Eastern Cooperative Oncology Group Trial HD06). Blood. 2003; Vol. 11, issue 11:26a. - PubMed
-
- Meyer RM, Gospodarowicz M, Connors JM, Pearcey RG, Wells WA, Winter JN. Final analysis of a randomized comparison of ABVD chemotherapy with a strategy that includes radiation therapy (RT) in patients with limited‐stage Hodgkin lymphoma (HL): NCIC CTG/ECOG HD.6. Blood. 2011; Vol. 118, issue 21.
-
- Meyer RM, Gospodarowicz MK, Connors JM, Pearcey RG, Bezjak A, Wells WA, et al. Randomized comparison of ABVD chemotherapy with a strategy that includes radiation therapy in patients with limited‐stage Hodgkin's lymphoma: National Cancer Institute of Canada Clinical Trials Group and the Eastern Cooperative Oncology Group. Journal of Clinical Oncology 2005;23(21):4634‐42. - PubMed
-
- Portlock CS. Clinical trials report. Comparison of ABVD chemotherapy and a regimen including radiation therapy in patients with limited‐stage non‐Hodgkin's lymphoma. Current Oncology Reports 2006;8(5):354‐7. - PubMed
Mexico B2H031 {published data only}
-
- Aviles A, Delgado S. A prospective clinical trial comparing chemotherapy, radiotherapy and combined therapy in the treatment of early stage Hodgkin's disease with bulky disease. Clinical & Laboratory Haematology 1998;20(2):95‐9. - PubMed
MSKCC trial #90‐44 {published data only}
-
- Straus DJ, Portlock CS, Qin J, Myers J, Zelenetz AD, Moskowitz C, et al. Results of a prospective randomized clinical trial of doxorubicin, bleomycin, vinblastine, and dacarbazine (ABVD) followed by radiation therapy (RT) versus ABVD alone for stages I, II, and IIIA nonbulky Hodgkin disease. Blood 2004;104(12):3483‐9. - PubMed
UK NCRI Rapid {published data only}
-
- Radford J. Update on the NCRI RAPID trial. 9th International Symposium on Hodgkin Lymphoma, Cologne, Germany 2013.
-
- Radford J, Barrington S, Counsell N, Pettengell R, Johnson P, Wimperis J, et al. Involved field radiotherapy versus no further treatment in patients with clinical stages IA and IIA Hodgkin lymphoma and a ‘negative’ PET scan after 3 cycles ABVD. Results of the UK NCRI RAPID trial. Blood. 2012; Vol. 120, issue 21.
-
- Radford J, Barrington S, Counsell N, Pettengell R, Johnson P, Wimperis J, et al. Prognostic performance of pre‐treatment EORTC, GHSG and IPI risk factors and post‐chemotherapy PET response in the UK NCRI RAPID TRIAL in early stage Hodgkin lymphoma (HL). Haematologica 2013;98 ( Suppl. 2 ):13.
-
- Radford J, Illidge T, Counsell N, Hancock B, Pettengell R, Johnson P, et al. Results of a trial of PET‐directed therapy for early‐stage Hodgkin’s lymphoma. New England Journal of Medicine 2015;372(17):1598‐607. - PubMed
-
- Radford J, O'Doherty M, Barrington S, Qian W, Patrick P, Coltart S, et al. Results of the 2nd planned interim analysis of the rapid trial (involved field radiotherapy versus no further treatment) in patients with clinical stages 1a and 2a Hodgkin lymphoma and a 'negative' FDG‐PET scan after 3 cycles ABVD. Blood 2008;112(11):143‐4.
References to studies excluded from this review
Andrieu 1999 {published data only}
-
- Andrieu JM, Jais JP, Escoffre‐Barbe M, Delwail V, Desablens B, Kiladjian JJ, et al. Bulky Hodgkin's disease (B‐HD): treatment with an initial 7 drug chemotherapy (CT) delivered over 12 weeks followed by high dose extended field irradiation (EF‐RT). Seven year results of the GOELAMS H90M multicentric randomized trial. Blood 1999;94(10 suppl.):abstract 528a.
Bonnet 2007 {published data only}
-
- Bonnet C, Fillet G, Mounier N, Ganem G, Molina TJ, Thiéblemont C, et al. CHOP alone compared with CHOP plus radiotherapy for localized aggressive lymphoma in elderly patients: a study by the Groupe d'Etude des Lymphomes de l'Adulte. Journal of Clinical Oncology 2007;25(7):787‐92. - PubMed
Brusamolino 1994 {published data only}
-
- Brusamolino E, Lazzarino M, Orlandi E, Canevari A, Morra E, Castelli G, et al. Early‐stage Hodgkin's disease: long‐term results with radiotherapy alone or combined radiotherapy and chemotherapy. Annals of Oncology 1994;5(suppl. 2):101‐6. - PubMed
Cheveresan 1998 {published data only}
-
- Cheveresan LF, Roth I, Balan M, Ionita H. Combined modality therapy in early stage Hodgkin's disease ‐ preliminary results of a clinical trial. Leukemia and Lymphoma 1998;29(suppl. 1):72.
Cimino 1990 {published data only}
-
- Cimino G. Chemotherapy alone for the treatment of early‐stage Hodgkin's disease. European Journal of Cancer 1990;26(11‐12):1115‐8. - PubMed
Cosset 1992 {published data only}
-
- Cosset JM, Henry‐Amar M, Meerwaldt JH, Carde P, Noordijk EM, Thomas J, et al. The EORTC trials for limited stage Hodgkin's disease. The EORTC Lymphoma Cooperative Group. European Journal of Cancer 1992;28A(11):1847‐50. - PubMed
Desablens 1999 {published data only}
-
- Desablens B, Jais JP, Lacotter‐Thierry L, Foussard C, Escoffre‐Barbe M, Moreau P, et al. Treatment of CS IA to IIIB non‐bulky Hodgkin's disease (NB‐HD) with 3 cycles of chemotherapy (CT) (ABVD vs EBVM) followed by high dose irradiation (RT). Results of the GOELAMS H90‐NM multicentre randomized trial. Blood 1999;94(10 suppl. 1):abstract 386a.
Dionet 1988 {published data only}
-
- Dionet C, Oberlin O, Habrand JL, Vilcoq J, Madelain M, Dutou L, et al. Initial chemotherapy and low‐dose radiation in limited fields in childhood Hodgkin's disease: results of a joint cooperative study by the French Society of Pediatric Oncology (SFOP) and Hôpital Saint‐Louis, Paris. International Journal of Radiation Oncology, Biology, Physics 1988;15(2):341‐6. - PubMed
Ferme 2005 {published data only}
-
- Ferme C, Diviné M, Vranovsky A, Morschhauser F, Bouabdallah R, Gabarre J, et al. Four ABVD and involvedfield radiotherapy in unfavorable supradiaphragmatic clinical stages (CS) III Hodgkin's lymphoma (HL): preliminary results of the EORTCGELA H9U trial [Abstract]. Blood 2005;106(11):abstract A‐813.
Friedmann 2014 {published data only}
-
- Friedman DL, Chen L, Wolden S, Buxton A, McCarten K, FitzGerald TJ, et al. Dose‐intensive response‐based chemotherapy and radiation therapy for children and adolescents with newly diagnosed intermediate‐risk Hodgkin lymphoma: a report from the Children’s Oncology Group study AHOD0031. Journal of Clinical Oncology 2014;32(32):3651‐8. - PMC - PubMed
Hirsch 1994 {published data only}
-
- Hirsch A. The effect of ABVD chemotherapy with and without mediastinal irradiation on pulmonary function and symptoms in early‐stage Hodgkin's disease. International Journal of Radiation Oncology, Biology, Physics 1994;30(suppl. 1):168.
Hirsch 1996 {published data only}
-
- Hirsch A, Vander EN, Straus DJ, Gomez EG, Leung D, Portlock CS, et al. Effect of ABVD chemotherapy with and without mantle or mediastinal irradiation on pulmonary function and symptoms in early‐stage Hodgkin's disease. Journal of Clinical Oncology 1996;14(4):1297‐305. - PubMed
Horning 1996 {published data only}
-
- Horning SJ, Bennett JM, Bartlett NL, Williams J, Neuberg D, Cassileth PA. 12 weeks of chemotherapy (STANFORD V) and involved field radiotherapy (RT) are highly effective for bulky and advanced stage Hodgkin's disease (HD): a limited institution ECOG pilot study. Blood 1996;88(10 Suppl (Pt 1)):673a.
Horning 2007 {published data only}
-
- Horning SJ, Hoppe RT, Advani RH, Breslin S, McCormick E, Allen J, et al. A prospective trial of involved field radiation (IFRT) + chemotherapy vs. extended field radiation (EFRT) for favorable Hodgkin's disease (HD): Long‐term follow‐up and implications for current combined modality. Haematologica 2007;92(Suppl. 5):53.
Kim 2003 {published data only}
-
- Kim HK, Silver B, Li S, Neuberg D, Mauch P. Hodgkin's disease in elderly patients (> or =60): clinical outcome and treatment strategies. International Journal of Radiation Oncology, Biology, Physics 2003;56(2):556‐60. - PubMed
Körholz 2004 {published data only}
-
- Körholz D, Claviez A, Hasenclever D, Kluge R, Hirsch W, Kamprad F, et al. The concept of the GPOH‐HD 2003 therapy study for pediatric Hodgkin's disease: evolution in the tradition of the DAL/GPOH studies. Klinische Pädiatrie 2004;216(3):150‐6. - PubMed
Kung 1993 {published data only}
-
- Kung FH, Behm FG, Cantor A, Falletta J, Ferree CR, Leventhal BG, et al. Abbreviated chemotherapy vs chemoradiotherapy in early stage Hodgkin's disease of childhood. Proceedings of the American Society of Clinical Oncology. 1993; Vol. 12:414.
Kung 2006 {published data only}
-
- Kung FH. POG 8625: A randomized trial comparing chemotherapy with chemoradiotherapy for children and adolescents with stages I, IIA, IIIA Hodgkin disease: A report from the children's oncology group. Journal of Pediatric Hematology/Oncology 2006;28(6):362‐8. - PubMed
Laskar 2004 {published data only}
-
- Laskar S, Gupta T, Vimal S, Muckaden MA, Saikia TK, Pai SK, et al. Consolidation radiation after complete remission in Hodgkin's disease following six cycles of doxorubicin, bleomycin, vinblastine, and dacarbazine chemotherapy: is there a need?. Journal of Clinical Oncology 2004;22(1):62‐8. - PubMed
Lemerle 1986 {published data only}
-
- Lemerle J, Oberlin O, Schaison G, Leverger G, Olive D, Duffilot B. Effectiveness of primary chemotherapy and low‐dose radiation (RT) in childhood Hodgkin's disease (HD) [abstract]. Proceedings of the American Society of Clinical Oncology. 1986.
Longo 1992 {published data only}
-
- Longo DL, DeVita VT. The use of combination chemotherapy in the treatment of early stage Hodgkin's disease. In: Vita VT, Helman S, Rosenberg SA editor(s). Important Advances in Oncology. Philadelphia: Lippincott Williams & Wilkins, 1992:155‐65. - PubMed
Meyer 2013 {published data only}
-
- Meyer RM. Radiation in early‐stage Hodgkin lymphoma. Clinical Advances in Hematology & Oncology 2013;11(3):162‐89. - PubMed
Nachman 2002 {published data only}
-
- Nachman JB, Sposto R, Herzog P, Gilchrist GS, Wolden SL, Thomson J, et al. Randomized comparison of low‐dose involved‐field radiotherapy and no radiotherapy for children with Hodgkin's disease who achieve a complete response to chemotherapy. Journal of Clinical Oncology 2002;20(18):3765‐71. - PubMed
Noordijk 2006 {published data only}
-
- Noordijk EM, Carde P, Dupouy N, Hagenbeek A, Krol AD, Kluin‐Nelemans JC, et al. Combined‐modality therapy for clinical stage I or II Hodgkin's lymphoma: long‐term results of the European Organisation for Research and Treatment of Cancer H7 randomized controlled trials. Journal of Clinical Oncology 2006;24(19):3128‐35. - PubMed
O'Dwyer 1984 {published data only}
-
- O'Dwyer PJ, Stewart MB, Wiernik PH. MOPP vs radiotherapy/MOPP for early‐stage Hodgkin's disease (HD) ‐ a six year follow‐up. 2nd International Conference on Malignant Lymphoma, Lugano, Switzerland. June 13, 1984; Vol. 16:46.
O'Dwyer 1985 {published data only}
-
- O'Dwyer PJ, Wiernik PH, Stewart MB, Slawson RG. Treatment of early stage Hodgkin's disease: a randomized trial of radiotherapy plus chemotherapy versus chemotherapy alone. In: Cavilli F, Bonadonna G, Rozencweig M editor(s). Malignant Lymphomas and Hodgkin's Disease: Experimental and Therapeutic Advances. Boston: Maritinus Nijhoff, 1985:329‐36.
Pavlovsky 1988 {published data only}
-
- Bloomfield CD, Pajak TF, Glicksman AS, Gottlieb AJ, Coleman M, Nissen NI, et al. Chemotherapy and combined modality therapy for Hodgkin's disease: a progress report on Cancer and Leukemia Group B studies. Cancer Treatment Reports 1982;66(4):835‐46. - PubMed
Pavlovsky 1997 {published data only}
-
- Pavlovsky S, Schvartzman E, Lastiri F, Magnasco H, Corrado C, Raslawski E, et al. Randomized trial of CVPP for three versus six cycles in favorable‐prognosis and CVPP versus AOPE plus radiotherapy in intermediate‐prognosis untreated Hodgkin's disease. Journal of Clinical Oncology 1997;15(7):2652‐8. - PubMed
Picardi 2007 {published data only}
-
- Picardi M, Renzo A, Pane F, Nicolai E, Pacelli R, Salvatore M, et al. Randomized comparison of consolidation radiation versus observation in bulky Hodgkin's lymphoma with post‐chemotherapy negative positron emission tomography scans. Leukemia & Lymphoma 2007;48(9):1721‐7. - PubMed
Radford 2002 {published data only}
-
- Radford JA, Cowan RA, Ryder WD, Johnson RJ, Bannerjee SS, Deakin DP, et al. Four weeks of VAPEC‐B chemotherapy before involved field radiotherapy minimises the relapse rate in early stage low‐risk Hodgkin's disease and is not associated with an excess of second malignancy. Annals of Oncology 2002;13(Suppl. 2):25.
Reinartz 2013 {published data only}
-
- Reinartz G, Eich HT. Does involved field radiotherapy improve survival for children with Hodgkin's lymphoma in complete remission after chemotherapy?. Strahlentherapie und Onkologie 2013 ;189(4):344‐6. - PubMed
Rüffer 1996 {published data only}
-
- Rüffer U, Brosteanu O, Sieber M, Koch T, Löffler M, Pfreundschuh M. Reduction of radiotherapy in early stage Hodgkin's disease: results of a randomized trial in patients ps I/II. Annals of Oncology 1996;7(Suppl. 3):49.
Rüffer 1998 {published data only}
-
- Rüffer U, Sieber M, Pfistner B, Tesch H, Engert A, Bredenfeld H, et al. Reduction of radiotherapy volume in intermediate Hodgkin's disease: Interim analysis of a randomized trial in patients CS I/II of the GHSG. Blood 1998;92(10 Suppl 1 (Pt 1)):abstract 626a.
Rüffer 1999 {published data only}
-
- Rüffer JU, Sieber M, Pfistner B, Tesch H, Engert A, Bredenfeld H, et al. For intermediate stage Hodgkin's disease extended field radiation after effective chemotherapy is obsolete: interim analysis of HD9 trial (GHSG). Blood 1999;94(10 Suppl. 1):528a.
Specht 1992 {published data only}
-
- Specht L, Carde P, Mauch P, Magrini SM, Santarelli MT. Radiotherapy versus combined modality in early stages. Annals of Oncology 1992;3(suppl. 4):77‐81. - PubMed
Straus 1989 {published data only}
-
- Straus DJ, Myers J, Lee BJ, Koziner B, Nisce LZ, Redman J. Limited chemotherapy and radiation therapy (RT) for early clinical stage (CS) Hodgkin's disease (HD). High complete remission (CR) percentage, disease free survival (DFS) and low toxicity. Blood 1989;74(7 Suppl. 1):239a.
Thistlethwaite 2007 {published data only}
-
- Thistlethwaite F, Qian W, Williams MV, Hancock BW, Hoskin P, Sun‐Mynt H, et al. Selection of patients for minimal initial chemotherapy (MIC); the impact of Hasenclever score on outcome in patients receiving MIC and involved field radiotherapy for clinical stage IA/IIA supra‐diaphragmatic Hodgkin lymphoma in the UK NCRI LY07 trial. Haematologica 2007;92(Suppl. 5):52.
Thomas 2004 {published data only}
-
- Thomas J, Ferme C, Noordijk EM, Rieux C, Divine M, Brice P, et al. Six cycles of ABVD + IFRT vs. four cycles of ABVD + IFRT vs. four cycles of BEACOPP + IFRT in unfavourable supradiaphragmatic clinical stages III Hodgkin's lymphoma: the EORTCGELA H9U randomized clinical trial (20982) in 808 patients [Abstract AE12]. European Journal of Haematology 2004;73(Supp 65):40.
Weiner 1997 {published data only}
-
- Weiner MA, Leventhal B, Brecher ML, Marcus RB, Cantor A, Gieser PW, et al. Randomized study of intensive MOPP‐ABVD with or without low‐dose total‐nodal radiation therapy in the treatment of stages IIB, IIIA2, IIIB, and IV Hodgkin's disease in pediatric patients: a Pediatric Oncology Group study. Journal of Clinical Oncology 1997;15(8):2769‐79. - PubMed
Wolden 2012 {published data only}
-
- Wolden SL, Chen L, Kelly KM, Herzog P, Gilchrist GS, Thomson J, et al. Long‐term results of CCG 5942: a randomized comparison of chemotherapy with and without radiotherapy for children with Hodgkin’s lymphoma—a report from the Children’s Oncology Group. Journal of Clinical Oncology 2012;30(26):3174‐80. - PMC - PubMed
References to ongoing studies
GHSG HD16 {published data only}
-
- GHSG. HD16 for early stages ‐ treatment optimization trial in the first‐line treatment of early stage Hodgkin lymphoma; treatment stratification by means of FDG‐PET. www.clinicaltrials.gov NCT00736320.
GHSG HD17 {published data only}
-
- GHSG. HD17 for intermediate stages treatment optimization trial in the firstline treatment of intermediate stage Hodgkin lymphoma. www.clinicaltrials.gov NCT01356680.
HD0801 {published data only}
-
- Fondazione Italiana Linfomi ONLUS. High‐dose chemotherapy and stem cell transplantation, in patients PET‐2 positive, after 2 courses of ABVD and comparison of RT versus no RT in PET‐2 negative patients (HD0801). www.clinicaltrials.gov NCT00784537.
Additional references
Adams 2004
-
- Adams MJ, Lipsitz SR, Colan SD, Tarbell NJ, Treves ST, Diller L, et al. Cardiovascular status in long‐term survivors of Hodgkin's disease treated with chest radiotherapy. Journal of Clinical Oncology 2004;22(15):3139‐48. - PubMed
Aleman 2003
-
- Aleman BM, Belt‐Dusebout AW, Klokman WJ, Van't Veer MB, Bartelink H, Leeuwen FE. Long‐term cause‐specific mortality of patients treated for Hodgkin's disease. Journal of Clinical Oncology 2003;21(18):3431‐9. - PubMed
Bhatia 2003
-
- Bhatia S, Yasui Y, Robison LL, Birch JM, Bogue MK, Diller L, et al. High risk of subsequent neoplasms continues with extended follow‐up of childhood Hodgkin's disease: report from the Late Effects Study Group. Journal of Clinical Oncology 2003;21(23):4386‐94. - PubMed
Canellos 2005
-
- Canellos GP. Chemotherapy alone for early Hodgkin's lymphoma: an emerging option. Journal of Clinical Oncology 2005;23(21):4574‐6. - PubMed
Carbone 1971
-
- Carbone PP, Kaplan HS, Musshoff K, Smithers DW, Tubiana M. Report of the Committee on Hodgkin's Disease Staging Classification. Cancer Research 1971;31(11):1860‐1. - PubMed
Chaber 2006
-
- Chaber BA, Amrein PC, Druker BJ, Michaelson MD, Mitsiades CS, Goss PF, et al. Antineoplastic agents. In: Brunton LL, Lazo JS, Parker KL editor(s). Goodman and Gillman's. The Pharmacological Basis of Therapeutics. 11. McGraw‐Hill, Medical Publishing Division, 2006:1380.
Cheson 2007
-
- Cheson BD, Pfistner B, Juweid ME, Gscoyne RD, Specht L, Horning SJ, et al. Revised response criteria for malignant lymphoma. Journal of Clinical Oncology 2007;25(5):579‐86. - PubMed
Connors 2001
-
- Connors JM, Noordijk EM, Horning SJ. Hodgkin's lymphoma: basing the treatment on the evidence. Hematology: American Society of Haematology Education Book. American Society of Hematology, 2001:178‐93. - PubMed
Connors 2005
-
- Connors JM. State‐of‐the‐art therapeutics: Hodgkin's lymphoma. Journal of Clinical Oncology 2005;23(26):6400‐8. - PubMed
Crump 2015
-
- Crump M, Herst J, Baldassarre F, Sussman J, MacEachern J, Hodgson D, et al. Evidence‐based focused review of the role of radiation therapy in the treatment of early‐stage Hodgkin lymphoma. Blood 2015;125(11):1708‐16. - PubMed
Deeks 2011
-
- Deeks JJ, Higgins JPT, Altman DG. Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
DeVita 1997
-
- DeVita VT Jr, Mauch PM, Harris NL. Hodgkin's disease. In: DeVita VT Jr, Hellmann S, Rosenberg SA editor(s). Cancer Principles and Practice of Oncology. 5th Edition. Philadelphia: Lippincott‐Raven, 1997:2242‐83.
Dickersin 1993
-
- Dickersin K, Min YI. NIH clinical trials and publication bias. Online Journal of Current Clinical Trials 1993;50:4967. - PubMed
Diehl 2005
-
- Diehl V, Re D, Josting A. Hodgkin's disease: clinical manifestations, staging, and therapy. In: Hoffman R, Benz EJ, Shattil SJ, Furie B, Cohen HJ editor(s). Hematology: Basic Principles and Practice. 4th Edition. Churchill Livingstone, 2005:1347‐78.
Dores 2002
-
- Dores GM, Metayer C, Curtis RE, Lynch CF, Clarke EA, Glimelius B, et al. Second malignant neoplasms among long‐term survivors of Hodgkin's disease: a population‐based evaluation over 25 years. Journal of Clinical Oncology 2002;20(16):3484‐94. - PubMed
Egger 1997
-
- Egger M, Zellweger‐Zahner T, Schneider M, Junker C, Lengeler C, Antes G. Language bias in randomised controlled trials published in English and German. Lancet 1997;350(9074):326‐9. - PubMed
Engert 2007
-
- Engert A, Franklin J, Eich HT, Brillant C, Sehlen S, Cartoni C, et al. Two cycles of doxorubicin, bleomycin, vinblastine, and dacarbazine plus extended‐field radiotherapy is superior to radiotherapy alone in early favorable Hodgkin's lymphoma: final results of the GHSG HD7 trial. Journal of Clinical Oncology 2007;25(23):3495‐502. - PubMed
Engert 2010
-
- Engert A, Plutschow A, Eich HT, Lohri A, Dorken B, Borchmann P, et al. Reduced treatment intensity in patients with early‐stage Hodgkin's lymphoma. The New England Journal of Medicine 2010;363(7):640‐52. - PubMed
Engert 2012
-
- Engert A, Haverkamp H, Kobe C, Markova J, Renner C, Ho A, et al. Reduced‐intensity chemotherapy and PET‐guided radiotherapy in patients with advanced stage Hodgkin's lymphoma (HD15 trial): a randomised, open‐label, phase 3 non‐inferiority trial. Lancet 2012;379(9828):1791‐9. [PUBMED: 22480758] - PubMed
Franklin 2005
Glaser 1996
-
- Glaser SL, Jarrett RF. The epidemiology of Hodgkin's disease. Baillière's Clinical Haematology 1996;9(3):401‐16. - PubMed
Green 2000
-
- Green DM, Hyland A, Barcos MP, Reynolds JA, Lee RJ, Hall BC, et al. Second malignant neoplasms after treatment for Hodgkin's disease in childhood or adolescence. Journal of Clinical Oncology 2000;18(7):1492‐9. - PubMed
Henderson 2016
Higgins 2011
-
- Higgins JPT, Deeks JJ. Chapter 7: Selecting studies and collecting data. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011.
Higgins 2011a
-
- Higgins JPT, Deeks JJ, Altman DG. Chapter 16: Special topics in statistics. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Higgins 2011b
-
- Higgins JPT, Altman DG. Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Juni 2002
-
- Juni P, Holenstein F, Sterne J, Bartlett C, Egger M. Direction and impact of language bias in meta‐analyses of controlled trials: empirical study. International Journal of Epidemiology 2002;31(1):115‐23. - PubMed
Klimm 2005
-
- Klimm B, Engert A, Diehl V. First‐line treatment of Hodgkin's lymphoma. Current Hematology Reports 2005;4(1):15‐22. - PubMed
Lefebvre 2011
-
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Lister 1989
-
- Lister TA, Crowther D, Sutcliffe SB, Glatstein E, Canellos GP, Young RC, et al. Report of a committee convened to discuss the evaluation and staging of patients with Hodgkin's disease: Cotswolds meeting. Journal of Clinical Oncology 1989;7(11):1630‐6. - PubMed
Maraldo 2015
-
- Maraldo MV, Giusti F, Vogelius IR, Lundemann M, Kaaij MA, Ramadan S, et al. Cardiovascular disease after treatment for Hodgkin's lymphoma: an analysis of nine collaborative EORTC‐LYSA trials. Lancet Haematology 2015;2(11):e492‐502. - PubMed
Mauch 1999
-
- Mauch PM, Armitage JO, Diehl V, Hoppe RT, Weiss LM. Hodgkin's Disease. 1st Edition. Philadelphia: Lippicott Williams & Wilkins, 1999.
McBride 2008
-
- McBride WH, Withers HR. Biologic basis of radiation therapy. In: Halperin EC, Perez CA, Brady LW editor(s). Principles and Practice of Radiation Oncology. 5th Edition. Philadelphia: Wolthers Kluwer, Lippincott Williams & Wilkin, 2008:76‐108.
Moher 2009
-
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. Journal of Clinical Epidemiology 2009;62(10):1006‐12. [PUBMED: 19631508] - PubMed
Mueller 1999
-
- Mueller NE, Gruffermann S. The epidemiology of Hodgkin's disease. In: Mauch PM, Armitage JO, Diehl V, Hoppe RT, Weiss LM editor(s). Hodgkin's Disease. 1st Edition. Philadelphia: Lippicott Williams & Wilkins, 1999:61‐78.
Ng 2002a
-
- Ng AK, Bernardo MP, Weller E, Backstrand KH, Silver B, Marcus KC, et al. Long‐term survival and competing causes of death in patients with early‐stage Hodgkin's disease treated at age 50 or younger. Journal of Clinical Oncology 2002;20(8):2101‐8. - PubMed
Ng 2002b
-
- Ng AK, Bernardo MV, Weller E, Backstrand K, Silver B, Marcus KC, et al. Second malignancy after Hodgkin disease treated with radiation therapy with or without chemotherapy: long‐term risks and risk factors. Blood 2002;100(6):1989‐96. - PubMed
Parkin 2005
-
- Parkin DM, Bray F, FerlayJ, Pisani P. Global Cancer Statistics, 2002. CA: A Cancer Journal for Clinicians 2005;55(2):74‐108. - PubMed
Parmar 1998
-
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta‐analyses of the published literature for survival endpoints. Statistics in Medicine 1998;17(24):2815‐34. - PubMed
Rancea 2013
RevMan 2014 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Sausville 2005
-
- Sausville EA, Longo DL. Principles of cancer treatment: surgery, chemotherapy and biologic therapy. In: Kasper DL, Fauci AS, Longo DL, Braunwald E, Hauser SL, Jameson JL editor(s). Harrison's Principles of Internal Medicine. 16. New York: McGraw‐Hill, Medical Publishing Division, 2005:464‐83.
Schaapveld 2015
-
- Schaapveld M, Aleman BM, Eggermond AM, Janus CP, Krol AD, Maazen RW, et al. Second cancer risk up to 40 years after treatment for Hodgkin’s lymphoma. New England Journal of Medicine 2015;373(26):2499‐511. - PubMed
Schünemann 2011
-
- Schünemann HJ, Oxman AD, Higgins JPT, Vist GE, Glasziou P, Guyatt GH. Chapter 11: Presenting results and 'Summary of findings tables'. In: Higgins JPT, Green S (editors). Cochrane Handbook of Systematic Reviews of Intervention. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Sickinger 2015
Sterne 2011
-
- Sterne JAC, Egger M, Moher D. Chapter 10: Addressing reporting biases. In: Higgins JPT, Green S (editors). Cochrane Handbook of Systematic Reviews of Intervention. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Swerdlow 2000
-
- Swerdlow AJ, Barber JA, Hudson GV, Cunningham D, Gupta RK, Hancock BW, et al. Risk of second malignancy after Hodgkin's disease in a collaborative British cohort: the relation to age at treatment. Journal of Clinical Oncology 2000;18(3):498‐509. - PubMed
Swerdlow 2003
-
- Swerdlow AJ. Epidemiology of Hodgkin's disease and non‐Hodgkin's lymphoma. European Journal of Nuclear Medicine & Molecular Imaging 2003;30 Suppl 1:S3‐12. - PubMed
Thomas 2002
-
- Thomas RK, Re D, Zander T, Wolf J, Diehl V. Epidemiology and etiology of Hodgkin's lymphoma. Annals of Oncology 2002;13 Suppl 4:147‐52. - PubMed
Tierney 2007
van Leeuwen 2000
-
- Leeuwen FE, Klokman WJ, Veer MB, Hagenbeek A, Krol AD, Vetter UA, et al. Long‐term risk of second malignancy in survivors of Hodgkin's disease treated during adolescence or young adulthood. Journal of Clinical Oncology 2000;18(3):487‐97. - PubMed
References to other published versions of this review
Herbst 2011
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

