Spatial computation of intratumoral T cells correlates with survival of patients with pancreatic cancer
- PMID: 28447602
- PMCID: PMC5414182
- DOI: 10.1038/ncomms15095
Spatial computation of intratumoral T cells correlates with survival of patients with pancreatic cancer
Abstract
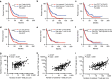
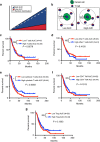
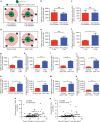
The exact nature and dynamics of pancreatic ductal adenocarcinoma (PDAC) immune composition remains largely unknown. Desmoplasia is suggested to polarize PDAC immunity. Therefore, a comprehensive evaluation of the composition and distribution of desmoplastic elements and T-cell infiltration is necessary to delineate their roles. Here we develop a novel computational imaging technology for the simultaneous evaluation of eight distinct markers, allowing for spatial analysis of distinct populations within the same section. We report a heterogeneous population of infiltrating T lymphocytes. Spatial distribution of cytotoxic T cells in proximity to cancer cells correlates with increased overall patient survival. Collagen-I and αSMA+ fibroblasts do not correlate with paucity in T-cell accumulation, suggesting that PDAC desmoplasia may not be a simple physical barrier. Further exploration of this technology may improve our understanding of how specific stromal composition could impact T-cell activity, with potential impact on the optimization of immune-modulatory therapies.
Conflict of interest statement
J.P.A. is an inventor of intellectual property owned by the University of California, Berkeley and licensed to Bristol Myers-Squibb. The remaining authors declare no competing financial interests.
Figures



References
-
- Schmitz-Winnenthal F. H. et al. High frequencies of functional tumour-reactive T cells in bone marrow and blood of pancreatic cancer patients. Cancer Res. 65, 10079–10087 (2005). - PubMed
-
- Fukunaga A. et al. CD8+ tumour-infiltrating lymphocytes together with CD4+ tumour-infiltrating lymphocytes and dendritic cells improve the prognosis of patients with pancreatic adenocarcinoma. Pancreas 28, e26-31 (2004). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

