Supervised dosing with a long-acting opioid medication in the management of opioid dependence
- PMID: 28447766
- PMCID: PMC6478186
- DOI: 10.1002/14651858.CD011983.pub2
Supervised dosing with a long-acting opioid medication in the management of opioid dependence
Abstract
Background: Opioid dependence (OD) is an increasing clinical and public health problem worldwide. International guidelines recommend opioid substitution treatment (OST), such as methadone and buprenorphine, as first-line medication treatment for OD. A negative aspect of OST is that the medication used can be diverted both through sale on the black market, and the unsanctioned use of medications. Daily supervised administration of medications used in OST has the advantage of reducing the risk of diversion, and may promote therapeutic engagement, potentially enhancing the psychosocial aspect of OST, but costs more and is more restrictive on the client than dispensing for off-site consumption.
Objectives: The objective of this systematic review is to compare the effectiveness of OST with supervised dosing relative to dispensing of medication for off-site consumption.
Search methods: We searched in Cochrane Drugs and Alcohol Group Specialised Register and Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, Embase, CINAHL, Web of Science from inception up to April 2016. Ongoing and unpublished studies were searched via ClinicalTrials.gov (www.clinicaltrials.gov) and World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (http://www.who.int/ictrp/en/).All searches included non-English language literature. We handsearched references on topic-related systematic reviews.
Selection criteria: Randomised controlled trials (RCTs), controlled clinical trials (CCTs), and prospective controlled cohort studies, involving people who are receiving OST (methadone, buprenorphine) and comparing supervised dosing with dispensing of medication to be consumed away from the dispensing point, usually without supervision.
Data collection and analysis: We used the standard methodological procedures expected by Cochrane.
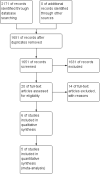
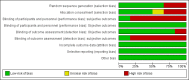
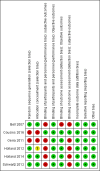
Main results: Six studies (four RCTs and two prospective observational cohort studies), involving 7999 participants comparing supervised OST treatment with unsupervised treatment, met the inclusion criteria. The risk of bias was generally moderate across trials, but the results reported on outcomes that we planned to consider were limited. Overall, we judged the quality of the evidence from very low to low for all the outcomes.We found no difference in retention at any duration with supervised compared to unsupervised dosing (RR 0.99, 95% CI 0.88 to 1.12, 716 participants, four trials, low-quality evidence) or in retention in the shortest follow-up period, three months (RR 0.94; 95% CI 0.84 to 1.05; 472 participants, three trials, low-quality evidence). Additional data at 12 months from one observational study found no difference in retention between groups (RR 0.94, 95% CI 0.77 to 1.14; n = 300).There was no difference in abstinence at the end of treatment (self-reported drug use) (67% versus 60%, P = 0.33, 293 participants, one trial, very low-quality evidence); and in diversion of medication (5% versus 2%, 293 participants, one trial, very low-quality evidence).Regarding our secondary outcomes, we did not found a difference in the incidence of adverse effects in the supervised compared to unsupervised control group (RR 0.63; 96% CI 0.10 to 3.86; 363 participants, two trials, very low-quality evidence). Data on severity of dependence were very limited (244 participants, one trial) and showed no difference between the two approaches. Data on deaths were reported in two studies. One trial reported two deaths in the supervised group (low-quality evidence), while in the cohort study all-cause mortality was found lower in regular supervision group (crude mortality rate 0.60 versus 0.81 per 100 person-years), although after adjustment insufficient evidence existed to suggest that regular supervision was protective (mortality rate ratio = 1.23, 95% CI = 0.67 to 2.27).No studies reported pain symptoms, drug craving, aberrant opioid-related behaviours, days of unsanctioned opioid use and overdose.
Authors' conclusions: Take-home medication strategies are attractive to treatment services due to lower costs, and place less restrictions on clients, but it is unknown whether they may be associated with increased risk of diversion and unsanctioned use of medication. There is uncertainty about the effects of supervised dosing compared with unsupervised medication due to the low and very low quality of the evidence for the primary outcomes of interest for this review. Data on defined secondary outcomes were similarly limited. More research comparing supervised and take-home medication strategies is needed to support decisions on the relative effectiveness of these strategies. The trials should be designed and conducted with high quality and over a longer follow-up period to support comparison of strategies at different stages of treatment. In particular, there is a need for studies assessing in more detail the risk of diversion and safety outcomes of using supervised OST to manage opioid dependence.
Conflict of interest statement
RS: no conflict of interest.
SV: no conflict of interest.
LG: no conflict of interest.
Figures


Update of
References
References to studies included in this review
Bell 2007 {published data only}
-
- Bell J, Shanahan M, Mutch C, Rea F, Ryan A, Batey R, et al. A randomized trial of effectiveness and cost‐effectiveness of observed versus unobserved administration of buprenorphine‐naloxone for heroin dependence. Addiction (Abingdon, England) 2007;102(12):1899‐907. - PubMed
Cousins 2016 {published data only}
-
- Cousins G, Boland F, Courtney B, Barry J, Lyons S, Fahey T. Risk of mortality on and off methadone substitutiontreatment in primary care: a national cohort study. Addiction 2016;111(1):73‐82. - PubMed
Gerra 2011 {published data only}
-
- Gerra G, Saenz E, Busse A, Maremmani I, Ciccocioppo R, Zaimovic A, et al. Supervised daily consumption, contingent take‐home incentive and non‐contingent take‐home in methadone maintenance. Progress in Neuro‐Psychopharmacology and Biological Psychiatry 2011;35(2):483‐9. - PubMed
Holland 2012 {published data only}
-
- Holland R, Matheson C, Anthony G, Roberts K, Priyardarshi S, Macrae A, et al. A pilot randomised controlled trial of brief versus twice weekly versus standard supervised consumption in patients on opiate maintenance treatment. Drug and Alcohol Review 2012;31(4):483‐91. - PubMed
Holland 2014 {published data only}
-
- Holland R, Maskrey V, Swift L, Notley C, Robinson A, Nagar J, et al. Treatment retention, drug use and social functioning outcomes in those receiving 3 months versus 1 month of supervised opioid maintenance treatment. Results from the Super C randomized controlled trial. Addiction (Abingdon, England) 2014;109(4):596‐607. - PubMed
References to studies excluded from this review
Amass 2001 {published data only}
-
- Amass L, Kamien JB, Mikulich SK. Thrice‐weekly supervised dosing with the combination buprenorphine‐naloxone tablet is preferred to daily supervised dosing by opioid‐dependent humans. Drug and Alcohol Dependence 2001;61(2):173‐81. - PubMed
Auriacombe 2004 {published data only}
-
- Auriacombe M, Fatseas M, Dubernet J, Daulouede JP, Tignol J. French field experience with buprenorphine. American Journal on Addiction 2004;13(S1):17‐28. - PubMed
Bell 2008 {published data only}
-
- Bell JR, Ryan A, Mutch C, Batey R, Rea F. Optimising the benefits of unobserved dose administration for stable opioid maintenance patients: Follow‐up of a randomised trial. Drug and Alcohol Dependence 2008;96:183–6. - PubMed
Carrieri 2014 {published data only}
Groshkova 2013 {published data only}
-
- Groshkova T, Metrebian N, Hallam C, Charles V, Martin A, Forzisi L, et al. Treatment expectations and satisfaction of treatment‐refractory opioid‐dependent patients in RIOTT, the Randomised Injectable Opiate Treatment Trial, the UK's first supervised injectable maintenance clinics. Drug and Alcohol Review 2013;32(6):566‐73. - PubMed
-
- Metrebian N, Groshkova T, Hellier J, Charles V, Martin A, Forzisi L, et al. Drug use, health and social outcomes of hard‐to‐treat heroin addicts receiving supervised injectable opiate treatment: secondary outcomes from the Randomized Injectable Opioid Treatment Trial (RIOTT). Addiction 2015;110(3):479‐90. [DOI: 10.1111/add.12748] - DOI - PubMed
Haasen 2007 {published data only}
-
- Haasen C, Verthein U, Degkwitz P, Berger J, Krausz M, Naber D. Heroin‐assisted treatment for opioid dependence: randomised controlled trial. British Journal of Psychiatry : the journal of mental science 2007;191(1):55‐62. - PubMed
Hutchinson 2000 {published data only}
-
- Hutchinson SJ, Taylor A, Gruer L, Barr C, Mills C, Elliott L, et al. One‐year follow‐up of opiate injectors treated with oral methadone in a GP‐centred programme. Addiction (Abingdon, England) 2000;95(7):1055‐68. - PubMed
Kakko 2003 {published data only}
-
- Kakko J, Svanborg KD, Kreek MJ, Heilig M. 1‐year retention and social function after buprenorphine‐assisted relapse prevention treatment for heroin dependence in Sweden: a randomised, placebo‐controlled trial. Lancet 2003;361(9358):662‐8. - PubMed
Krook 2002 {published data only}
-
- Krook AL, Brors O, Dahlberg J, Grouff K, Magnus P, Roysamb E, et al. A placebo‐controlled study of high dose buprenorphine in opiate dependents waiting for medication‐assisted rehabilitation in Oslo, Norway. Addiction (Abingdon, England) 2002;97(5):533‐42. - PubMed
Lintzeris 2006 {published data only}
-
- Lintzeris N, Strang J, Metrebian N, Byford S, Hallam C, Lee S, et al. Methodology for the Randomised Injecting Opioid Treatment Trial (RIOTT): evaluating injectable methadone and injectable heroin treatment versus optimised oral methadone treatment in the UK. Harm Reduction Journal 2006;3:28. - PMC - PubMed
Lintzeris 2013 {published data only}
-
- Lintzeris N, Leung SY, Dunlop AJ, Larance B, White N, Rivas GR, et al. A randomised controlled trial of sublingual buprenorphine‐naloxone film versus tablets in the management of opioid dependence. Drug and Alcohol Dependence 2013;131(1‐2):119‐26. - PubMed
Strang 2010 {published data only}
-
- Strang J, Metrebian N, Lintzeris N, Potts L, Carnwath T, Mayet S, et al. Supervised injectable heroin or injectable methadone versus optimised oral methadone as treatment for chronic heroin addicts in England after persistent failure in orthodox treatment (RIOTT): a randomised trial. Lancet 2010;375(9729):1885‐95. - PubMed
Suzuki 2014 {published data only}
-
- Suzuki J, Matthews ML, Brick D, Nguyen MT, Wasan AD, Jamison RN, et al. Implementation of a collaborative care management program with buprenorphine in primary care: a comparison between opioid‐dependent patients and patients with chronic pain using opioids nonmedically. Journal of Opioid Management 2014;10(3):159‐68. - PMC - PubMed
Additional references
Amato 2011
Bell 2014
-
- Bell J. Pharmacologicalmaintenance treatments ofopiate addiction. British Journal of ClinicalPharmacology 2014;77(2):253–63. [DOI: ]
Cai 2010
-
- Cai R, Crane, E, Poneleit, K, PaulozzI L. Emergency department visits involving nonmedical use of selected prescription drugs in the United States, 2004‐2008. Journal of Pain & Palliative Care Pharmacotherapy 2010;24(3):293‐7. - PubMed
Cicero 2005
-
- Cicero TJ. Diversion and abuse of methadone prescribed for pain management. JAMA 2005;293:297–8. - PubMed
Cleeland 2009
-
- Cleeland CS. The Brief Pain Inventory. User Guide. Houston,Texas: The University of Texas, Anderson Cancer Center, 2009.
Dale‐Perera 2015
-
- Dale‐Perera A, Alam F, Barker P. Opioid‐dependence treatment in the era of recovery: insights from a UK survey of physicians, patients and out‐of‐treatment opioid users. Journal of Substance Use 2015;20(5):354‐62.
Dolan 2015
-
- Dolan K, Mehrjerdi ZA. Medication assisted treatment of opioid dependence: a review of the evidence. Australian National Council on Drugs 2015.
EPOC 2016
-
- Effective Practice and Organization of Care Cochrane Group. EPOC Resources for Cochrane Authors. http://epoc.cochrane.org/epoc‐specific‐resources‐review‐authors (accessed 22 February, 2016).
Fischer 2012
-
- Fischer B, Argento E. Prescription opioid related misuse, harms, diversion and interventions in Canada: a review. Pain Physician 2012;5:ES191–203. - PubMed
Fountain 2000
-
- Fountain J, Strang J, Gossop M, Farrell M, Griffiths P. Diversion of prescribed drugs by drug users in treatment: analysis of the UK market and new data from London. Addiction 2000;95:393–406. - PubMed
Franklin 2009
-
- Franklin GM, Rahman EA, Turner JA, Daniell WE, Fulton‐Kehoe D. Opioid use for chronic low back pain: A prospective, population‐based study among injured workers in Washington state, 2002‐2005. Clinical Journal of Pain 2009;25(9):743–51. - PubMed
Gowing 2014
-
- Gowing L, Ali R, Dunlop A, Farrell M, Lintzeris N. National Guidelines for Medication‐Assisted Treatment of Opioid Dependence. Print ISBN: 978‐1‐74241‐944‐2; Online ISBN: 978‐1‐74241‐945‐9 2014.
Higgins 2011
-
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011.
IOM 2011
Johannes 2010
Kissin 2013
Larance 2011
-
- Larance B, Degenhardt L, Lintzeris N, Bell J, Winstock A, Dietze P, et al. Post‐marketing surveillance of buprenorphine‐naloxone in Australia: diversion, injectionand adherence with supervised dosing. Drug Alcohol Dependence 2011;1(118(2‐3)):265‐73. [doi: 10.1016/j.drugalcdep.2011.04.002.] - PubMed
Ling 2011
Maxwell 2011
-
- Maxwell JC. The prescription drug epidemic in the United States: A perfect storm. Drug and Alcohol Review 2011;30(3):264‐70. - PubMed
McLellan 2000
-
- McLellan AT, Lewis DC, O’Brien CP, Kleber HD. Drug dependence, a chronic medical illness: implications for treatment, insurance, and outcomes evaluation. JAMA 2000;284(13):1689‐9. - PubMed
Melzack 1975
-
- Melzack R. The McGill Pain Questionnaire: major properties and scoring methods. Pain 1975;1(3):277‐99. - PubMed
NICE 2007
-
- NICE. Technology appraisal guidance‐Methadone and buprenorphine for the management of opioid dependence 2007. https://www.nice.org.uk/guidance/ta114. - PubMed
Notley 2013
-
- Notley CN, Holland R, Nagar J, Maskrey V, Kouimtsidis C. Regaining control: the patient experience of supervised compared to unsupervised consumption in opiate substitution treatment. Drug and Alcohol Review 2014;33(1):64‐70. [10.1111/ dar.12079] - PubMed
RevMan 2014 [Computer program]
-
- Copenaghen: The Nordic Cochrane Centre. Review Manager (RevMan) Version 5.3. The Cochrane Collaboration, 2014.
Ritter 2005
-
- Ritter A, Natalie R. The relationship between take‐away methadone policies and methadone diversion. Drug and Alcohol Review 2005;24(4):347–52. - PubMed
Roxburgh 2011
-
- Roxburgh A, Bruno R, Larance B, Burns L. Prescription of opioid analgesics and related harms in Australia. Medical Journal of Australia 2011;195(5):280‐4. - PubMed
Rudd 2016
-
- Rudd RA, Aleshire N, Zibbell JE, Gladden M. Increases in Drug and Opioid Overdose Deaths‐‐United States, 2000‐2014. MMWR. Morbidity and Mortality Weekly Report 2016;64((50‐51)):1378‐82. - PubMed
SAMHSA 2015 a
-
- Substance Abuse and Mental Health Services Administration, Center for Behavioral Health Statistics and Quality. The DAWN Report: Highlights of the 2011 Drug Abuse Warning Network (DAWN) Findings on Drug‐Related Emergency Department Visits. Accessed December 10, 2015. Available from: https://www.samhsa.gov/data/sites/default/files/DAWN127/DAWN127/sr127‐DA... accessed December 10, 2015. - PubMed
SAMSHA 2015 b
-
- SAMHSA 2015. Behavioral Health Trends in the United States: Results from the 2014 National Survey on Drug Use and Health. HHS Publication No. SMA 15‐4927, NSDUH Series H‐50. Accessed January 2016.
Schubert 2013
Seymour 2003
-
- Seymour A, Black M, Jay J, Cooper G, Weir C, Oliver J. The role of methadone in drug related deaths in the west of Scotland. Addiction 2003;98:995‐1002. - PubMed
Sigmon 2006
-
- Sigmon S. Characterizing the emerging population of prescription opioid abusers. American Journal on Addictions / American Academy of Psychiatrists in Alcoholism and Addictions 2006;15:208–12. - PubMed
UNODC 2014
-
- United Nations Office on Drugs and Crime (UNODC). World Drug Report 2015. www.unodc.org/documents/wdr2015/World_Drug_Report_2015.pdf.
UNODC 2016
-
- United Nations Office on Drugs and Crime. World Drug Report 2016. United Nations publication 2016.
Varenbut 2007
WHO 2004
-
- World Health Organization. Substitution maintenance therapy in the management of opioid dependence and HIV/AIDS prevention, 2004. WHO/UNODC/UNAIDS position paper 2004.
WHO 2009
-
- WHO 2009. Guidelines for the Psychosocially Assisted Pharmacological Treatment of Opioid Dependence. http://www.who.int/hiv/pub/idu/opioid/en/ 2009; Vol. ISBN 978 92 4 154754 3. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

