Isolation and Characterization of Mesenchymal Stromal Cells from Human Umbilical Cord and Fetal Placenta
- PMID: 28447991
- PMCID: PMC5564456
- DOI: 10.3791/55224
Isolation and Characterization of Mesenchymal Stromal Cells from Human Umbilical Cord and Fetal Placenta
Abstract
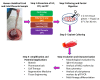
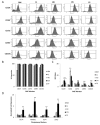
The human umbilical cord (UC) and placenta are non-invasive, primitive and abundant sources of mesenchymal stromal cells (MSCs) that have increasingly gained attention because they do not pose any ethical or moral concerns. Current methods to isolate MSCs from UC yield low amounts of cells with variable proliferation potentials. Since UC is an anatomically-complex organ, differences in MSC properties may be due to the differences in the anatomical regions of their isolation. In this study, we first dissected the cord/placenta samples into three discrete anatomical regions: UC, cord-placenta junction (CPJ), and fetal placenta (FP). Second, two distinct zones, cord lining (CL) and Wharton's jelly (WJ), were separated. The explant culture technique was then used to isolate cells from the four sources. The time required for the primary culture of cells from the explants varied depending on the source of the tissue. Outgrowth of the cells occurred within 3 - 4 days of the CPJ explants, whereas growth was observed after 7 - 10 days and 11 - 14 days from CL/WJ and FP explants, respectively. The isolated cells were adherent to plastic and displayed fibroblastoid morphology and surface markers, such as CD29, CD44, CD73, CD90, and CD105, similarly to bone marrow (BM)-derived MSCs. However, the colony-forming efficiency of the cells varied, with CPJ-MSCs and WJ-MSCs showing higher efficiency than BM-MSCs. MSCs from all four sources differentiated into adipogenic, chondrogenic, and osteogenic lineages, indicating that they were multipotent. CPJ-MSCs differentiated more efficiently in comparison to other MSC sources. These results suggest that the CPJ is the most potent anatomical region and yields a higher number of cells, with greater proliferation and self-renewal capacities in vitro. In conclusion, the comparative analysis of the MSCs from the four sources indicated that CPJ is a more promising source of MSCs for cell therapy, regenerative medicine, and tissue engineering.
References
-
- Upadhyay RK. Role of regeneration in tissue repairing and therapies. J Regen Med Tissue Eng. 2015;4(1):1.
-
- English K, Mahon BP, Wood KJ. Mesenchymal stromal cells; role in tissue repair, drug discovery and immune modulation. Curr Drug Deliv. 2014;11(5):561–571. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous