Systematic Review and Network Meta-Analysis of Bevacizumab Plus First-Line Topotecan-Paclitaxel or Cisplatin-Paclitaxel Versus Non-Bevacizumab-Containing Therapies in Persistent, Recurrent, or Metastatic Cervical Cancer
- PMID: 28448304
- PMCID: PMC5499964
- DOI: 10.1097/IGC.0000000000001000
Systematic Review and Network Meta-Analysis of Bevacizumab Plus First-Line Topotecan-Paclitaxel or Cisplatin-Paclitaxel Versus Non-Bevacizumab-Containing Therapies in Persistent, Recurrent, or Metastatic Cervical Cancer
Abstract
Objective: Despite advances in cervical cancer prevention and diagnosis, outcomes for patients given a diagnosis of advanced and recurrent disease are poor. In the GOG240 trial, the addition of bevacizumab to paclitaxel-topotecan or paclitaxel-cisplatin has been shown to prolong survival compared with paclitaxel-topotecan or paclitaxel-cisplatin in patients with persistent, recurrent, or metastatic disease. However, standards of care vary between regions and countries. The purpose of this systematic review and network meta-analysis was to enable a comparison between bevacizumab + chemotherapy with multiple monotherapy or combination chemotherapy regimens in the treatment for women with advanced, recurrent, or persistent cervical cancer.
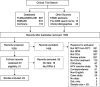
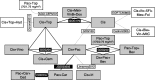
Methods/materials: A systematic literature review was conducted to identify randomized or nonrandomized controlled trials of patients with recurrent, persistent, or metastatic cervical cancer published in English from 1999 to 2015. A feasibility study was performed to assess the heterogeneity of the trials, and a network meta-analysis was conducted. Fixed- and random-effects models were fitted to calculate the hazard ratio for overall survival (OS) for all pairwise comparisons and ranking of all interventions.
Results: Twenty-three studies (19 trials) met inclusion criteria and were included in the review. Sample sizes ranged from 69 to 452, and median patient age ranged from 45 to 53 years. There was a trend toward prolonged OS with cisplatin-paclitaxel-bevacizumab and topotecan-paclitaxel-bevacizumab compared with all non-bevacizumab-containing therapies. Cisplatin-paclitaxel-bevacizumab had the highest probability of being the most efficacious compared with all regimens (68.1%), and cisplatin monotherapy had the lowest (0%).
Conclusions: The results of this network meta-analysis show that bevacizumab in combination with paclitaxel-topotecan or paclitaxel-cisplatin is likely to prolong OS over other non-bevacizumab-containing chemotherapies (eg, paclitaxel-carboplatin), which were not included in the GOG240 trial. In patients with advanced, persistent, and recurrent cervical cancer, cisplatin-paclitaxel-bevacizumab and topotecan-paclitaxel-bevacizumab showed the highest efficacy in all regimens investigated in this analysis.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of topotecan for ovarian cancer.Health Technol Assess. 2001;5(28):1-110. doi: 10.3310/hta5280. Health Technol Assess. 2001. PMID: 11701100
-
Topotecan, pegylated liposomal doxorubicin hydrochloride, paclitaxel, trabectedin and gemcitabine for advanced recurrent or refractory ovarian cancer: a systematic review and economic evaluation.Health Technol Assess. 2015 Jan;19(7):1-480. doi: 10.3310/hta19070. Health Technol Assess. 2015. PMID: 25626481 Free PMC article.
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of paclitaxel, docetaxel, gemcitabine and vinorelbine in non-small-cell lung cancer.Health Technol Assess. 2001;5(32):1-195. doi: 10.3310/hta5320. Health Technol Assess. 2001. PMID: 12065068
-
Topotecan, pegylated liposomal doxorubicin hydrochloride and paclitaxel for second-line or subsequent treatment of advanced ovarian cancer: a systematic review and economic evaluation.Health Technol Assess. 2006 Mar;10(9):1-132. iii-iv. doi: 10.3310/hta10090. Health Technol Assess. 2006. PMID: 16545208
-
Pembrolizumab with Carboplatin and Paclitaxel Versus Alternative Systemic Treatments Recommended for the First-Line Treatment of Recurrent/Metastatic Head and Neck Cancer: An Indirect Treatment Comparison.Adv Ther. 2025 Jun;42(6):2690-2707. doi: 10.1007/s12325-025-03144-4. Epub 2025 Apr 24. Adv Ther. 2025. PMID: 40272712
Cited by
-
Targeting the Tie2-αvβ3 integrin axis with bi-specific reagents for the inhibition of angiogenesis.BMC Biol. 2018 Aug 17;16(1):92. doi: 10.1186/s12915-018-0557-9. BMC Biol. 2018. PMID: 30119679 Free PMC article.
-
Efficacy and safety of intensity Modulated Radiation therapy combined with Concurrent Chemoradiotherapy in the treatment of Recurrent Cervical Cancer.Pak J Med Sci. 2023 Jul-Aug;39(4):1062-1067. doi: 10.12669/pjms.39.4.6784. Pak J Med Sci. 2023. PMID: 37492330 Free PMC article.
-
Paclitaxel alters the microvascular network in the central and peripheral nervous system of rats with chemotherapy-induced painful peripheral neuropathy.J Peripher Nerv Syst. 2024 Dec;29(4):537-554. doi: 10.1111/jns.12660. Epub 2024 Oct 22. J Peripher Nerv Syst. 2024. PMID: 39434652 Free PMC article.
-
Efficacy and Safety of Apatinib for the Treatment of Advanced or Recurrent Cervical Cancer: A Single-Arm Meta-Analysis Among Chinese Patients.Front Pharmacol. 2022 Aug 11;13:843905. doi: 10.3389/fphar.2022.843905. eCollection 2022. Front Pharmacol. 2022. PMID: 36034824 Free PMC article.
-
Efficacy and Safety of Atezolizumab as a PD-L1 Inhibitor in the Treatment of Cervical Cancer: A Systematic Review.Biomedicines. 2024 Jun 11;12(6):1291. doi: 10.3390/biomedicines12061291. Biomedicines. 2024. PMID: 38927498 Free PMC article. Review.
References
-
- Cervical Cancer UK. Cervical Cancer Incidence Statistics. 2014. Available at: http://www.cancerresearchuk.org/health-professional/cancer-statistics/st.... Accessed January 17, 2016.
-
- World Health Organization. GLOBOCAN 2012: Estimated Cancer Incidence, Mortality and Prevalence Worldwide in 2012. 2012. Available at: http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx. Accessed October 15, 2014.
-
- World Health Organization. Human Papillomavirus (HPV) and Cervical Cancer. Fact sheet no. 380. Available at: http://www.who.int/mediacentre/factsheets/fs380/en/. Accessed January 17, 2016.
-
- US National Library of Medicine. Cervical Cancer. 2015. Available at: https://www.nlm.nih.gov/medlineplus/cervicalcancer.html. Accessed September 11, 2014.
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

