Dysregulation of INF2-mediated mitochondrial fission in SPOP-mutated prostate cancer
- PMID: 28448495
- PMCID: PMC5426793
- DOI: 10.1371/journal.pgen.1006748
Dysregulation of INF2-mediated mitochondrial fission in SPOP-mutated prostate cancer
Abstract
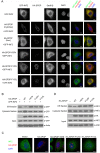
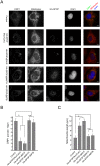
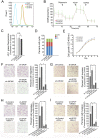
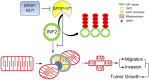
Next-generation sequencing of the exome and genome of prostate cancers has identified numerous genetic alternations. SPOP (Speckle-type POZ Protein) was one of the most frequently mutated genes in primary prostate cancer, suggesting SPOP is a potential driver of prostate cancer development and progression. However, how SPOP mutations contribute to prostate cancer pathogenesis remains poorly understood. SPOP acts as an adaptor protein of the CUL3-RBX1 E3 ubiquitin ligase complex that generally recruits substrates for ubiquitination and subsequent degradation. ER-localized isoform of the formin protein inverted formin 2 (INF2) mediates actin polymerization at ER-mitochondria intersections and facilitates DRP1 recruitment to mitochondria, which is a critical step in mitochondrial fission. Here, we revealed that SPOP recognizes a Ser/Thr (S/T)-rich motif in the C-terminal region of INF2 and triggers atypical polyubiquitination of INF2. These ubiquitination modifications do not lead to INF2 instability, but rather reduces INF2 localization in ER and mitochondrially associated DRP1 puncta formation, therefore abrogates its ability to facilitate mitochondrial fission. INF2 mutant escaping from SPOP-mediated ubiquitination is more potent in prompting mitochondrial fission. Moreover, prostate cancer-associated SPOP mutants increase INF2 localization in ER and promote mitochondrial fission, probably through a dominant-negative effect to inhibit endogenous SPOP. Moreover, INF2 is important for SPOP inactivation-induced prostate cancer cell migration and invasion. These findings reveal novel molecular events underlying the regulation of INF2 function and localization, and provided insights in understanding the relationship between SPOP mutations and dysregulation of mitochondrial dynamics in prostate cancer.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures





References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

