When do correlations increase with firing rates in recurrent networks?
- PMID: 28448499
- PMCID: PMC5426798
- DOI: 10.1371/journal.pcbi.1005506
When do correlations increase with firing rates in recurrent networks?
Abstract
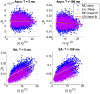
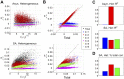
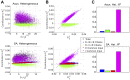
A central question in neuroscience is to understand how noisy firing patterns are used to transmit information. Because neural spiking is noisy, spiking patterns are often quantified via pairwise correlations, or the probability that two cells will spike coincidentally, above and beyond their baseline firing rate. One observation frequently made in experiments, is that correlations can increase systematically with firing rate. Theoretical studies have determined that stimulus-dependent correlations that increase with firing rate can have beneficial effects on information coding; however, we still have an incomplete understanding of what circuit mechanisms do, or do not, produce this correlation-firing rate relationship. Here, we studied the relationship between pairwise correlations and firing rates in recurrently coupled excitatory-inhibitory spiking networks with conductance-based synapses. We found that with stronger excitatory coupling, a positive relationship emerged between pairwise correlations and firing rates. To explain these findings, we used linear response theory to predict the full correlation matrix and to decompose correlations in terms of graph motifs. We then used this decomposition to explain why covariation of correlations with firing rate-a relationship previously explained in feedforward networks driven by correlated input-emerges in some recurrent networks but not in others. Furthermore, when correlations covary with firing rate, this relationship is reflected in low-rank structure in the correlation matrix.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures





References
-
- Kay SM. Fundamentals of Statistical Signal Processing, Volume 1: Estimation Theory. Prentice Hall PTR; 1993.
-
- Dayan P, Abbott LF. Theoretical Neuroscience: Computational and Mathematical Modeling of Neural Systems. Taylor & Francis; 2001.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

