Clinical efficacy and safety of mesenchymal stem cell transplantation for osteoarthritis treatment: A meta-analysis
- PMID: 28448518
- PMCID: PMC5407776
- DOI: 10.1371/journal.pone.0175449
Clinical efficacy and safety of mesenchymal stem cell transplantation for osteoarthritis treatment: A meta-analysis
Abstract
Purpose: The aim of this study was to evaluate the therapeutic efficacy and safety of mesenchymal stem cells (MSCs) for the treatment of patients with knee osteoarthritis (OA).
Materials: We performed a meta-analysis of relevant published clinical studies. An electronic search was conducted for randomized controlled trials (RCTs) of MSC-based therapy in knee OA. The visual analogue scale (VAS), International Knee Documentation Committee (IKDC) form, Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC), Lequesne algofunctional indices (Lequesne), Lysholm knee scale (Lysholm), Tegner activity scale (Tegner) and adverse events (AEs) were evaluated.
Results: Eleven eligible trials with 582 knee OA patients were included in the present meta-analysis. We demonstrated that MSC treatment could significantly decrease VAS and increase IKDC scoresafter a 24-month follow-up compared with controls (P<0.05). MSC therapy also showed significant decreases in WOMAC and Lequesne scores after the 12-month follow-up (P<0.01). Analysis of Lysholm (24-month) and Tegner (12- and 24-month) scores also demonstrated favorable results for MSC treatment (P<0.05).
Conclusion: Overall, MSC transplantation treatment was shown to be safe and has great potential as an efficacious clinical therapy for patients with knee OA.
Conflict of interest statement
Figures




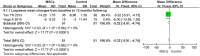


References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

