Vitamin D3-induced hypercalcemia increases carbon tetrachloride-induced hepatotoxicity through elevated oxidative stress in mice
- PMID: 28448545
- PMCID: PMC5407844
- DOI: 10.1371/journal.pone.0176524
Vitamin D3-induced hypercalcemia increases carbon tetrachloride-induced hepatotoxicity through elevated oxidative stress in mice
Abstract
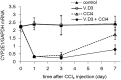
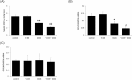
The aim of this study was to determine whether calcium potentiates acute carbon tetrachloride (CCl4) -induced toxicity. Elevated calcium levels were induced in mice by pre-treatment with cholecalciferol (vitamin D3; V.D3), a compound that has previously been shown to induce hypercalcemia in human and animal models. As seen previously, mice injected with CCl4 exhibited increased plasma levels of alanine aminotransferase, aspartate aminotransferase, and creatinine; transient body weight loss; and increased lipid peroxidation along with decreased total antioxidant power, glutathione, ATP, and NADPH. Pre-treatment of these animals with V.D3 caused further elevation of the values of these liver functional markers without altering kidney functional markers; continued weight loss; a lower lethal threshold dose of CCl4; and enhanced effects on lipid peroxidation and total antioxidant power. In contrast, exposure to V.D3 alone had no effect on plasma markers of liver or kidney damage or on total antioxidant power or lipid peroxidation. The potentiating effect of V.D3 was positively correlated with elevation of hepatic calcium levels. Furthermore, direct injection of CaCl2 also enhanced CCl4-induced hepatic injury. Since CaCl2 induced hypercalcemia transiently (within 3 h of injection), our results suggest that calcium enhances the CCl4-induced hepatotoxicity at an early stage via potentiation of oxidative stress.
Conflict of interest statement
Figures









Similar articles
-
Hepatoprotective and antioxidant effects of single clove garlic against CCl4-induced hepatic damage in rabbits.BMC Complement Altern Med. 2017 Aug 17;17(1):411. doi: 10.1186/s12906-017-1916-8. BMC Complement Altern Med. 2017. PMID: 28818066 Free PMC article.
-
Suppressive Effect of Kampo Formula "Juzen-taiho-to" on Carbon Tetrachloride-Induced Hepatotoxicity in Mice.Biol Pharm Bull. 2016;39(9):1564-7. doi: 10.1248/bpb.b16-00421. Biol Pharm Bull. 2016. PMID: 27582337
-
Carbon tetrachloride-induced hepatic and renal damages in rat: inhibitory effects of cacao polyphenol.Biosci Biotechnol Biochem. 2015;79(10):1669-75. doi: 10.1080/09168451.2015.1039481. Epub 2015 May 21. Biosci Biotechnol Biochem. 2015. PMID: 25996516
-
Effect of pretreatment with artichoke extract on carbon tetrachloride-induced liver injury and oxidative stress.Exp Toxicol Pathol. 2008 Sep;60(6):475-80. doi: 10.1016/j.etp.2008.04.014. Epub 2008 Jun 25. Exp Toxicol Pathol. 2008. PMID: 18583118
-
Antioxidative and chemopreventive effects of Nephrolepis biserrata against carbon tetrachloride (CCl4)-induced oxidative stress and hepatic dysfunction in rats.Pharm Biol. 2015 Jan;53(1):31-9. doi: 10.3109/13880209.2014.909502. Epub 2014 Sep 22. Pharm Biol. 2015. PMID: 25243876
Cited by
-
Turning the backbone into an ankylosed concrete-like structure: Case report.Medicine (Baltimore). 2018 Apr;97(15):e0278. doi: 10.1097/MD.0000000000010278. Medicine (Baltimore). 2018. PMID: 29642148 Free PMC article.
-
Comparing distress of mouse models for liver damage.Sci Rep. 2020 Nov 13;10(1):19814. doi: 10.1038/s41598-020-76391-w. Sci Rep. 2020. PMID: 33188220 Free PMC article.
References
-
- Recknagel RO. Carbon tetrachloride hepatotoxicity. Pharmacol Rev. 1967;19(2):145–208. Epub 1967/06/01. - PubMed
-
- Weber LW, Boll M, Stampfl A. Hepatotoxicity and mechanism of action of haloalkanes: carbon tetrachloride as a toxicological model. Crit Rev Toxicol. 2003;33(2):105–36. Epub 2003/04/24. doi: 10.1080/713611034 - DOI - PubMed
-
- Recknagel RO, Glende EA Jr., Dolak JA, Waller RL. Mechanisms of carbon tetrachloride toxicity. Pharmacol Ther. 1989;43(1):139–54. Epub 1989/01/01. - PubMed
-
- Wong FW, Chan WY, Lee SS. Resistance to carbon tetrachloride-induced hepatotoxicity in mice which lack CYP2E1 expression. Toxicol Appl Pharmacol. 1998;153(1):109–18. Epub 1999/01/06. doi: 10.1006/taap.1998.8547 - DOI - PubMed
-
- Manibusan MK, Odin M, Eastmond DA. Postulated carbon tetrachloride mode of action: a review. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2007;25(3):185–209. Epub 2007/09/01. doi: 10.1080/10590500701569398 - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

