Characterization of physiological defects in adult SIRT6-/- mice
- PMID: 28448551
- PMCID: PMC5407791
- DOI: 10.1371/journal.pone.0176371
Characterization of physiological defects in adult SIRT6-/- mice
Abstract
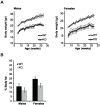
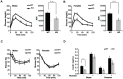
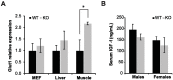
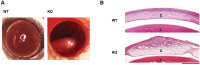
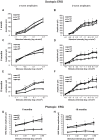
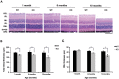
The NAD+-dependent SIRT6 deacetylase was shown to be a major regulator of lifespan and healthspan. Mice deficient for SIRT6 develop a premature aging phenotype and metabolic defects, and die before four weeks of age. Thus, the effect of SIRT6 deficiency in adult mice is unknown. Here we show that SIRT6-/- mice in mixed 129/SvJ/BALB/c background reach adulthood, allowing examination of SIRT6-related metabolic and developmental phenotypes in adult mice. In this mixed background, at 200 days of age, more than 80% of the female knock-out mice were alive whereas only 10% of male knock-out mice survived. In comparison to their wild-type littermates, SIRT6 deficient mice have reduced body weight, increased glucose uptake and exhibit an age-dependent progressive impairment of retinal function accompanied by thinning of retinal layers. Together, these results demonstrate a role for SIRT6 in metabolism and age-related ocular changes in adult mice and suggest a gender specific regulation of lifespan by SIRT6.
Conflict of interest statement
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

