The impact of anti-drug antibodies on drug concentrations and clinical outcomes in rheumatoid arthritis patients treated with adalimumab, etanercept, or infliximab: Results from a multinational, real-world clinical practice, non-interventional study
- PMID: 28448562
- PMCID: PMC5407581
- DOI: 10.1371/journal.pone.0175207
The impact of anti-drug antibodies on drug concentrations and clinical outcomes in rheumatoid arthritis patients treated with adalimumab, etanercept, or infliximab: Results from a multinational, real-world clinical practice, non-interventional study
Erratum in
-
Correction: The impact of anti-drug antibodies on drug concentrations and clinical outcomes in rheumatoid arthritis patients treated with adalimumab, etanercept, or infliximab: Results from a multinational, real-world clinical practice, non-interventional study.PLoS One. 2017 Jun 5;12(6):e0179308. doi: 10.1371/journal.pone.0179308. eCollection 2017. PLoS One. 2017. PMID: 28582423 Free PMC article.
Abstract
Objective: To assess the incidence of anti-drug antibodies (ADA) in patients with rheumatoid arthritis (RA) treated with the TNF inhibitors etanercept (ETN), adalimumab (ADL), or infliximab (IFX), and determine the potential relationship with trough drug concentration, efficacy, and patient-reported outcomes.
Methods: This multi-national, non-interventional, cross-sectional study (NCT01981473) enrolled adult patients with RA treated continuously for 6-24 months with ETN, ADL, or IFX. ADA and trough drug concentrations were measured by independent assays ≤2 days before the next scheduled dose. Efficacy measurements included Disease Activity Score 28-joint count (DAS28), low disease activity (LDA), remission, and erythrocyte sedimentation rate (ESR). Targeted medical histories of injection site/infusion reactions, serum sickness, and thromboembolic events were collected.
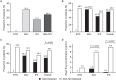
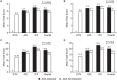
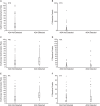
Results: Baseline demographics of the 595 patients (ETN: n = 200; ADL: n = 199; IFX: n = 196) were similar across groups. The mean duration of treatment was 14.6, 13.5, and 13.1 months for ETN, ADL, and IFX, respectively. All ETN-treated patients tested negative for ADA, whereas 31.2% and 17.4% patients treated with ADL and IFX, respectively, tested positive. In ADL- or IFX-treated patients, those with ADA had significantly lower trough drug concentrations. There were negative correlations between trough drug levels and both CRP and ESR in ADL- and IFX-treated patients. DAS28-ESR LDA and remission rates were higher in patients without ADA. The rate of targeted medical events reported was low.
Conclusion: ADA were detected in ADL- and IFX-treated but not ETN-treated patients. Patients without ADA generally showed numerically better clinical outcomes than those with ADA.
Trial registration: This study was registered on www.ClinicalTrials.gov (NCT01981473).
Conflict of interest statement
Figures


References
-
- Kourilovitch M, Galarza-Maldonado C, Ortiz-Prado E (2014) Diagnosis and classification of rheumatoid arthritis. J Autoimmun 48–49: 26–30. - PubMed
-
- Ma VY, Chan L, Carruthers KJ (2014) Incidence, prevalence, costs, and impact on disability of common conditions requiring rehabilitation in the United States: stroke, spinal cord injury, traumatic brain injury, multiple sclerosis, osteoarthritis, rheumatoid arthritis, limb loss, and back pain. Arch Phys Med Rehabil 95: 986–995.e981. 10.1016/j.apmr.2013.10.032 - DOI - PMC - PubMed
-
- AbbVie Inc (2002) HUMIRA (adalimumab) injection, Prescribing Information.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

