Inhibitory effects of fenretinide metabolites N-[4-methoxyphenyl]retinamide (MPR) and 4-oxo-N-(4-hydroxyphenyl)retinamide (3-keto-HPR) on fenretinide molecular targets β-carotene oxygenase 1, stearoyl-CoA desaturase 1 and dihydroceramide Δ4-desaturase 1
- PMID: 28448568
- PMCID: PMC5407626
- DOI: 10.1371/journal.pone.0176487
Inhibitory effects of fenretinide metabolites N-[4-methoxyphenyl]retinamide (MPR) and 4-oxo-N-(4-hydroxyphenyl)retinamide (3-keto-HPR) on fenretinide molecular targets β-carotene oxygenase 1, stearoyl-CoA desaturase 1 and dihydroceramide Δ4-desaturase 1
Abstract
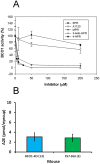
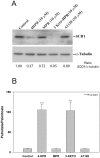
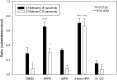
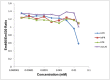
The therapeutic capacity of fenretinide (N-[4-hydroxyphenyl] retinamide; 4-HPR) has been demonstrated for several conditions, including cancer, obesity, diabetes, and ocular disease. Yet, the mechanisms of action for its pleiotropic effects are still undefined. We hypothesized that investigation of two of the major physiological metabolites of fenretinide, N-[4-methoxyphenyl]retinamide (MPR) and 4-oxo-N-(4-hydroxyphenyl)retinamide (3-keto-HPR), might begin to resolve the multifaceted effects of this synthetic retinoid. We analyzed the effects of fenretinide, MPR, 3-keto-HPR, and the non-retinoid RBP4 ligand A1120, on the activity of known targets of fenretinide, stearoyl-CoA desaturase 1 (SCD1) and dihydroceramide Δ4-desaturase 1 (DES1) in ARPE-19 cells, and purified recombinant mouse beta-carotene oxygenase 1 (BCO1) in vitro. Lipids and retinoids were extracted and quantified by liquid chromatography-mass spectrometry and reversed phase HPLC, respectively. The data demonstrate that while fenretinide is an inhibitor of the activities of these three enzymes, that 3-keto-HPR is a more potent inhibitor of all three enzymes, potentially mediating most of the in vivo beneficial effects of fenretinide. However, while MPR does not affect SCD1 and DES1 activity, it is a potent specific inhibitor of BCO1. We conclude that a deeper understanding of the mechanisms of action of fenretinide and its metabolites provides new avenues for therapeutic specificity. For example, administration of 3-keto-HPR instead of fenretinide may be preferential if inhibition of SCD1 or DES1 activity is the goal (cancer), while MPR may be better for BCO1 modulation (carotenoid metabolism). Continued investigation of fenretinide metabolites in the context of fenretinide's various therapeutic uses will begin to resolve the pleotropic nature of this compound.
Conflict of interest statement
Figures
References
-
- Moon RC, Thompson HJ, Becci PJ, Grubbs CJ, Gander RJ, Newton DL, et al. (1979) N-(4-Hydroxyphenyl)retinamide, a new retinoid for prevention of breast cancer in the rat. Cancer Res 39: 1339–1346. - PubMed
-
- Hultin TA, May CM, Moon RC (1986) N-(4-hydroxyphenyl)-all-trans-retinamide pharmacokinetics in female rats and mice. Drug Metab Dispos 14: 714–717. - PubMed
-
- Mehta RG, Moon RC, Hawthorne M, Formelli F, Costa A (1991) Distribution of fenretinide in the mammary gland of breast cancer patients. Eur J Cancer 27: 138–141. - PubMed
-
- Hansen LA, Sigman CC, Andreola F, Ross SA, Kelloff GJ, De Luca LM (2000) Retinoids in chemoprevention and differentiation therapy. Carcinogenesis 21: 1271–1279. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

