Management of chronic Hepatitis C at a primary health clinic in the high-burden context of Karachi, Pakistan
- PMID: 28448576
- PMCID: PMC5407611
- DOI: 10.1371/journal.pone.0175562
Management of chronic Hepatitis C at a primary health clinic in the high-burden context of Karachi, Pakistan
Erratum in
-
Correction: Management of chronic Hepatitis C at a primary health clinic in the high-burden context of Karachi, Pakistan.PLoS One. 2017 Jun 22;12(6):e0180286. doi: 10.1371/journal.pone.0180286. eCollection 2017. PLoS One. 2017. PMID: 28640872 Free PMC article.
Abstract
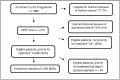
Background: The burden of hepatitis C (HCV) infection in Pakistan is among the highest in the world, with a reported national HCV prevalence of 6.7% in 2014. In specific populations, such as in urban communities in Karachi, the prevalence is suspected to be higher. Interferon-free treatment for chronic HCV infection (CHC) could allow scale up, simplification and decentralization of treatment to such communities. We present an interim analysis over the course of February-December 2015 of an interferon-free, decentralised CHC programme in the community clinic in Machar Colony, Karachi, Pakistan.
Design: A retrospective analysis of a treatment cohort.
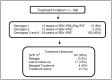
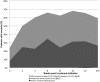
Results: There were 1,089 patients included in this analysis. Aspartate to platelet ratio index score was used to prioritize patients in terms of treatment initiation, with 242 patients placed in high priority for treatment and 202 starting treatment as scheduled. 169 patients started HCV treatment with Sofosbuvir-Ribavirin regimen according to HCV genotype over the course of 2015: of these, 35% had Hemoglobin reductions below 11.0 g/dl during the treatment course. Among the 153 patients (85%) with genotype 3 HCV infection, 84% of patients achieved sustained virologic response at 12 weeks following treatment completion (SVR 12).
Conclusion: Outcomes of HCV treatment with all oral combination in an integrated, decentralized model of care for CHC in a primary care setting, using simplified diagnostic and treatment algorithms, are comparable to the outcomes achieved in clinical trial settings for Sofosbuvir-based regimens. Our results suggest the feasibility and the pertinence if including interferon-free treatment regimens in the national programme, at both provincial and national levels.
Conflict of interest statement
Figures
References
-
- World Health Organization. Guidelines for the screening, care and treatment of persons with Hepatitis C infection. Geneva (CH); WHO; 2014. 122 p - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

