Effect of Neoadjuvant Chemotherapy Followed by Surgical Resection on Survival in Patients With Limited Metastatic Gastric or Gastroesophageal Junction Cancer: The AIO-FLOT3 Trial
- PMID: 28448662
- PMCID: PMC5824287
- DOI: 10.1001/jamaoncol.2017.0515
Effect of Neoadjuvant Chemotherapy Followed by Surgical Resection on Survival in Patients With Limited Metastatic Gastric or Gastroesophageal Junction Cancer: The AIO-FLOT3 Trial
Abstract
Importance: Surgical resection has a potential benefit for patients with metastatic adenocarcinoma of the stomach and gastroesophageal junction.
Objective: To evaluate outcome in patients with limited metastatic disease who receive chemotherapy first and proceed to surgical resection.
Design, setting, and participants: The AIO-FLOT3 (Arbeitsgemeinschaft Internistische Onkologie-fluorouracil, leucovorin, oxaliplatin, and docetaxel) trial is a prospective, phase 2 trial of 252 patients with resectable or metastatic gastric or gastroesophageal junction adenocarcinoma. Patients were enrolled from 52 cancer care centers in Germany between February 1, 2009, and January 31, 2010, and stratified to 1 of 3 groups: resectable (arm A), limited metastatic (arm B), or extensive metastatic (arm C). Data cutoff was January 2012, and the analysis was performed in March 2013.
Interventions: Patients in arm A received 4 preoperative cycles of fluorouracil, leucovorin, oxaliplatin, and docetaxel (FLOT) followed by surgery and 4 postoperative cycles. Patients in arm B received at least 4 cycles of neoadjuvant FLOT and proceeded to surgical resection if restaging (using computed tomography and magnetic resonance imaging) showed a chance of margin-free (R0) resection of the primary tumor and at least a macroscopic complete resection of the metastatic lesions. Patients in arm C were offered FLOT chemotherapy and surgery only if required for palliation. Patients received a median (range) of 8 (1-15) cycles of FLOT.
Main outcomes and measures: The primary end point was overall survival.
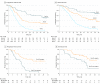
Results: In total, 238 of 252 patients (94.4%) were eligible to participate. The median (range) age of participants was 66 (36-79) years in arm A (n = 51), 63 (28-79) years in arm B (n = 60), and 65 (23-83) years in arm C (n = 127). Patients in arm B (n = 60) had only retroperitoneal lymph node involvement (27 patients [45%]), liver involvement (11 [18.3%]), lung involvement (10 [16.7%]), localized peritoneal involvement (4 [6.7%]), or other (8 [13.3%]) incurable sites. Median overall survival was 22.9 months (95% CI, 16.5 to upper level not achieved) for arm B, compared with 10.7 months (95% CI, 9.1-12.8) for arm C (hazard ratio, 0.37; 95% CI, 0.25-0.55) (P < .001). The response rate for arm B was 60% (complete, 10%; partial, 50%), which is higher than the 43.3% for arm C. In arm B, 36 of 60 patients (60%) proceeded to surgery. The median overall survival was 31.3 months (95% CI, 18.9-upper level not achieved) for patients who proceeded to surgery and 15.9 months (95% CI, 7.1-22.9) for the other patients.
Conclusions and relevance: Patients with limited metastatic disease who received neoadjuvant chemotherapy and proceeded to surgery showed a favorable survival. The AIO-FLOT3 trial provides a rationale for further randomized clinical trials.
Trial registration: clinicaltrials.gov identifier: NCT00849615.
Conflict of interest statement
Figures

References
-
- Hartgrink HH, Putter H, Klein Kranenbarg E, Bonenkamp JJ, van de Velde CJ; Dutch Gastric Cancer Group . Value of palliative resection in gastric cancer. Br J Surg. 2002;89(11):1438-1443. - PubMed
-
- Yoshida M, Ohtsu A, Boku N, et al. . Long-term survival and prognostic factors in patients with metastatic gastric cancers treated with chemotherapy in the Japan Clinical Oncology Group (JCOG) study. Jpn J Clin Oncol. 2004;34(11):654-659. - PubMed
-
- Lee JH, Paik YH, Lee JS, et al. . Candidates for curative resection in advanced gastric cancer patients who had equivocal para-aortic lymph node metastasis on computed tomographic scan. Ann Surg Oncol. 2006;13(9):1163-1167. - PubMed
-
- Cheon SH, Rha SY, Jeung HC, et al. . Survival benefit of combined curative resection of the stomach (D2 resection) and liver in gastric cancer patients with liver metastases. Ann Oncol. 2008;19(6):1146-1153. - PubMed
-
- Fujitani K, Yang HK, Mizusawa J, et al. ; REGATTA study investigators . Gastrectomy plus chemotherapy versus chemotherapy alone for advanced gastric cancer with a single non-curable factor (REGATTA): a phase 3, randomised controlled trial. Lancet Oncol. 2016;17(3):309-318. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

