Oral administration of methysticin improves cognitive deficits in a mouse model of Alzheimer's disease
- PMID: 28448946
- PMCID: PMC5406548
- DOI: 10.1016/j.redox.2017.04.024
Oral administration of methysticin improves cognitive deficits in a mouse model of Alzheimer's disease
Abstract
Introduction: There is increasing evidence for the involvement of chronic inflammation and oxidative stress in the pathogenesis of Alzheimer's disease (AD). Nuclear factor erythroid 2-related factor 2 (Nrf2) is an anti-inflammatory transcription factor that regulates the oxidative stress defense. Our previous experiments demonstrated that kavalactones protect neuronal cells against Amyloid β (Aβ)-induced oxidative stress in vitro by Nrf2 pathway activation. Here, we tested an in vivo kavalactone treatment in a mouse model of AD.
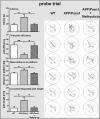
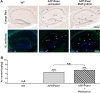
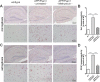
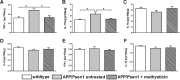
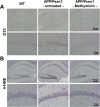
Methods: The kavalactone methysticin was administered once a week for a period of 6 months to 6 month old transgenic APP/Psen1 mice by oral gavage. Nrf2 pathway activation was measured by methysticin treatment of ARE-luciferase mice, by qPCR of Nrf2-target genes and immunohistochemical detection of Nrf2. Aβ burden was analyzed by CongoRed staining, immunofluorescent detection and ELISA. Neuroinflammation was assessed by immunohistochemical stainings for microglia and astrocytes. Pro-inflammatory cytokines in the hippocampus was determined by Luminex multi-plex assays. The hippocampal oxidative damage was detected by oxyblot technique and immunohistochemical staining against DT3 and 4-HNE. The cognitive ability of mice was evaluated using Morris water maze.
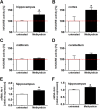
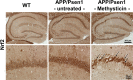
Results: Methysticin treatment activated the Nrf2 pathway in the hippocampus and cortex of mice. The Aβ deposition in brains of methysticin-treated APP/Psen1 mice was not altered compared to untreated mice. However, methysticin treatment significantly reduced microgliosis, astrogliosis and secretion of the pro-inflammatory cytokines TNF-α and IL-17A. In addition, the oxidative damage of hippocampi from APP/Psen1 mice was reduced by methysticin treatment. Most importantly, methysticin treatment significantly attenuated the long-term memory decline of APP/Psen1 mice.
Conclusion: In summary, these findings show that methysticin administration activates the Nrf2 pathway and reduces neuroinflammation, hippocampal oxidative damage and memory loss in a mouse model of AD. Therefore, kavalactones might be suitable candidates to serve as lead compounds for the development of a new class of neuroprotective drugs.
Keywords: Alzheimer's disease; Astrogliosis; Kava kava; Kavalactone; Methysticin; Neuroinflammation; Nrf2; Oxidative stress.
Copyright © 2017 The Authors. Published by Elsevier B.V. All rights reserved.
Figures

References
-
- Rodriguez J.J., Verkhratsky A. Neuroglial roots of neurodegenerative diseases? Mol. Neurobiol. 2011;43:87–96. - PubMed
-
- Wruck C.J., Gotz M.E., Herdegen T., Varoga D., Brandenburg L.O., Pufe T. Kavalactones protect neural cells against amyloid beta peptide-induced neurotoxicity via extracellular signal-regulated kinase 1/2-dependent nuclear factor erythroid 2-related factor 2 activation. Mol. Pharmacol. 2008;73:1785–1795. - PubMed
-
- Wruck C.J., Claussen M., Fuhrmann G., Romer L., Schulz A., Pufe T., Waetzig V., Peipp M., Herdegen T., Gotz M.E. Luteolin protects rat PC12 and C6 cells against MPP+ induced toxicity via an ERK dependent Keap1-Nrf2-ARE pathway. J. Neural Transm. Suppl. 2007:57–67. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

