Synthetic, Switchable Enzymes
- PMID: 28448969
- PMCID: PMC5526678
- DOI: 10.1159/000464443
Synthetic, Switchable Enzymes
Abstract
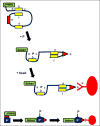
The construction of switchable, radiation-controlled, aptameric enzymes - "swenzymes" - is, in principle, feasible. We propose a strategy to make such catalysts from 2 (or more) aptamers each selected to bind specifically to one of the substrates in, for example, a 2-substrate reaction. Construction of a combinatorial library of candidate swenzymes entails selecting a set of a million aptamers that bind one substrate and a second set of a million aptamers that bind the second substrate; the aptamers in these sets are then linked pairwise by a linker, thus bringing together the substrates. In the presence of the substrates, some linked aptamer pairs catalyze the reaction when exposed to external energy in the form of a specific frequency of low-intensity, nonionizing electromagnetic or acoustic radiation. Such swenzymes are detected via a separate product-capturing aptamer that changes conformation on capturing the product; this altered conformation allows it (1) to bind to every potential swenzyme in its vicinity (thereby giving a higher probability of capture to the swenzymes that generate the product) and (2) to bind to a sequence on a magnetic bead (thereby permitting purification of the swenzyme plus product-capturing aptamer by precipitation). Attempts to implement the swenzyme strategy may help elucidate fundamental problems in enzyme catalysis.
Keywords: Aptamer; Catalyst; Catalytic antibody; Enzyme; Light; Radiation; Ribozyme; Sound; Synthetic biology.
© 2017 S. Karger AG, Basel.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures


References
-
- Agarwal PK, Schultz C, Kalivretenos A, Ghosh B, Broedel SE., Jr Engineering a hyper-catalytic enzyme by photoactivated conformation modulation. J Phys Chem Lett. 2012;3:1142–1146.
-
- An S, Kumar R, Sheets ED, Benkovic SJ. Reversible compartmentalization of de novo purine biosynthetic complexes in living cells. Science. 2008;320:103–106. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

