Mutations in THAP11 cause an inborn error of cobalamin metabolism and developmental abnormalities
- PMID: 28449119
- PMCID: PMC5886234
- DOI: 10.1093/hmg/ddx157
Mutations in THAP11 cause an inborn error of cobalamin metabolism and developmental abnormalities
Abstract
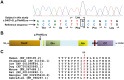
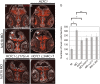
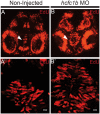
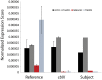
CblX (MIM309541) is an X-linked recessive disorder characterized by defects in cobalamin (vitamin B12) metabolism and other developmental defects. Mutations in HCFC1, a transcriptional co-regulator which interacts with multiple transcription factors, have been associated with cblX. HCFC1 regulates cobalamin metabolism via the regulation of MMACHC expression through its interaction with THAP11, a THAP domain-containing transcription factor. The HCFC1/THAP11 complex potentially regulates genes involved in diverse cellular functions including cell cycle, proliferation, and transcription. Thus, it is likely that mutation of THAP11 also results in biochemical and other phenotypes similar to those observed in patients with cblX. We report a patient who presented with clinical and biochemical phenotypic features that overlap cblX, but who does not have any mutations in either MMACHC or HCFC1. We sequenced THAP11 by Sanger sequencing and discovered a potentially pathogenic, homozygous variant, c.240C > G (p.Phe80Leu). Functional analysis in the developing zebrafish embryo demonstrated that both THAP11 and HCFC1 regulate the proliferation and differentiation of neural precursors, suggesting important roles in normal brain development. The loss of THAP11 in zebrafish embryos results in craniofacial abnormalities including the complete loss of Meckel's cartilage, the ceratohyal, and all of the ceratobranchial cartilages. These data are consistent with our previous work that demonstrated a role for HCFC1 in vertebrate craniofacial development. High throughput RNA-sequencing analysis reveals several overlapping gene targets of HCFC1 and THAP11. Thus, both HCFC1 and THAP11 play important roles in the regulation of cobalamin metabolism as well as other pathways involved in early vertebrate development.
© The Author 2017. Published by Oxford University Press. All rights reserved. For Permissions, please email: journals.permissions@oup.com.
Figures


Similar articles
-
Hcfc1b, a zebrafish ortholog of HCFC1, regulates craniofacial development by modulating mmachc expression.Dev Biol. 2014 Dec 1;396(1):94-106. doi: 10.1016/j.ydbio.2014.09.026. Epub 2014 Oct 2. Dev Biol. 2014. PMID: 25281006 Free PMC article.
-
THAP11F80L cobalamin disorder-associated mutation reveals normal and pathogenic THAP11 functions in gene expression and cell proliferation.PLoS One. 2020 Jan 6;15(1):e0224646. doi: 10.1371/journal.pone.0224646. eCollection 2020. PLoS One. 2020. PMID: 31905202 Free PMC article.
-
An X-linked cobalamin disorder caused by mutations in transcriptional coregulator HCFC1.Am J Hum Genet. 2013 Sep 5;93(3):506-14. doi: 10.1016/j.ajhg.2013.07.022. Am J Hum Genet. 2013. PMID: 24011988 Free PMC article.
-
Dissecting the role of vitamin B12 metabolism in craniofacial development through analysis of clinical phenotypes and model organism discoveries.Differentiation. 2025 Mar-Apr;142:100831. doi: 10.1016/j.diff.2024.100831. Epub 2024 Dec 10. Differentiation. 2025. PMID: 39676000 Review.
-
Methionine dependence in tumor cells: The potential role of cobalamin and MMACHC.Mol Genet Metab. 2021 Mar;132(3):155-161. doi: 10.1016/j.ymgme.2021.01.006. Epub 2021 Jan 13. Mol Genet Metab. 2021. PMID: 33487542 Review.
Cited by
-
Missense and nonsense mutations of the zebrafish hcfc1a gene result in contrasting mTor and radial glial phenotypes.Gene. 2023 May 15;864:147290. doi: 10.1016/j.gene.2023.147290. Epub 2023 Feb 17. Gene. 2023. PMID: 36804358 Free PMC article.
-
Ronin overexpression induces cerebellar degeneration in a mouse model of ataxia.Dis Model Mech. 2021 Jun 1;14(6):dmm044834. doi: 10.1242/dmm.044834. Epub 2021 Jun 24. Dis Model Mech. 2021. PMID: 34165550 Free PMC article.
-
Novel exon-skipping variant disrupting the basic domain of HCFC1 causes intellectual disability without metabolic abnormalities in both male and female patients.J Hum Genet. 2021 Jul;66(7):717-724. doi: 10.1038/s10038-020-00892-9. Epub 2021 Jan 30. J Hum Genet. 2021. PMID: 33517344
-
A case of methylmalonic acidemia and homocysteinemia cblX type with negative tandem mass spectrometry testing.Zhejiang Da Xue Xue Bao Yi Xue Ban. 2021 Dec 25;50(6):795-798. doi: 10.3724/zdxbyxb-2021-0262. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2021. PMID: 35347920 Free PMC article. English.
-
Combining newborn metabolic and DNA analysis for second-tier testing of methylmalonic acidemia.Genet Med. 2019 Apr;21(4):896-903. doi: 10.1038/s41436-018-0272-5. Epub 2018 Sep 13. Genet Med. 2019. PMID: 30209273 Free PMC article.
References
-
- Gérard M., Morin G., Bourillon A., Colson C., Mathieu S., Rabier D., Billette de Villemeur T., Ogier de Baulny H., Benoist J.F. (2015) Multiple congenital anomalies in two boys with mutation in HCFC1 and cobalamin disorder. Eur. J. Med. Genet., 58, 148–153. - PubMed
-
- Knez J., Piluso D., Bilan P., Capone J.P. (2006) Host cell factor-1 and E2F4 interact via multiple determinants in each protein. Mol. Cell. Biochem., 288, 79–90. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

