Operational tolerance in kidney transplantation and associated biomarkers
- PMID: 28449211
- PMCID: PMC5508347
- DOI: 10.1111/cei.12981
Operational tolerance in kidney transplantation and associated biomarkers
Abstract
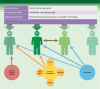
In the 1960s, our predecessors won a historical battle against acute rejection and ensured that transplantation became a common life-saving treatment. In parallel with this success, or perhaps because of it, we lost the battle for long-lived transplants, being overwhelmed with chronic immune insults and the toxicities of immunosuppression. It is likely that current powerful treatments block acute rejection, but at the same time condemn the few circulating donor cells that would have been able to elicit immunoregulatory host responses towards the allograft. Under these conditions, spontaneously tolerant kidney recipients - i.e. patients who maintain allograft function in the absence of immunosuppression - are merely accidents; they are scarce, mysterious and precious. Several teams pursue the goal of finding a biomarker that would guide us towards the 'just right' level of immunosuppression that avoids rejection while leaving some space for donor immune cells. Some cellular assays are attractive because they are antigen-specific, and provide a comprehensive view of immune responses toward the graft. These seem to closely follow patient regulatory capacities. However, these tests are cumbersome, and require abundant cellular material from both donor and recipient. The latest newcomers, non-antigen-specific recipient blood transcriptomic biomarkers, offer the promise that a practicable and simple signature may be found that overcomes the complexity of a system in which an infinite number of individual cell combinations can lead possibly to graft acceptance. Biomarker studies are as much an objective - identifying tolerant patients, enabling tolerance trials - as a means to deciphering the underlying mechanisms of one of the most important current issues in transplantation.
Keywords: biomarker; cellular assays; immunoquiescence; kidney transplantation; operational tolerance.
© 2017 British Society for Immunology.
Figures

References
-
- Billingham RE, Brent L, Medawar PB. Actively acquired tolerance of foreign cells. Nature 1953; 172:603–6. - PubMed
-
- Meier‐Kriesche HU, Schold JD, Srinivas TR, Kaplan B. Lack of improvement in renal allograft survival despite a marked decrease in acute rejection rates over the most recent era. Am J Transplant 2004; 4:378–83. - PubMed
-
- Braconnier P, Del Marmol V, Broeders N et al Combined introduction of anti‐IL2 receptor antibodies, mycophenolic acid and tacrolimus: effect on malignancies after renal transplantation in a single‐centre retrospective cohort study. Nephrol Dial Transplant 2012; 27:2547–53. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

