Long-term plasticity of corticostriatal synapses is modulated by pathway-specific co-release of opioids through κ-opioid receptors
- PMID: 28449351
- PMCID: PMC5556153
- DOI: 10.1113/JP274190
Long-term plasticity of corticostriatal synapses is modulated by pathway-specific co-release of opioids through κ-opioid receptors
Abstract
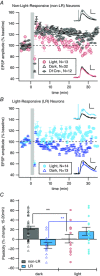
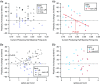
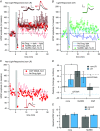
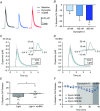
Key points: Both endogenous opioids and opiate drugs of abuse modulate learning of habitual and goal-directed actions, and can also modify long-term plasticity of corticostriatal synapses. Striatal projection neurons of the direct pathway co-release the opioid neuropeptide dynorphin which can inhibit dopamine release via κ-opioid receptors. Theta-burst stimulation of corticostriatal fibres produces long-term potentiation (LTP) in striatal projection neurons when measured using whole-cell patch recording. Optogenetic activation of direct pathway striatal projection neurons inhibits LTP while reducing dopamine release. Because the endogenous release of opioids is activity dependent, this modulation of synaptic plasticity represents a negative feedback mechanism that may limit runaway enhancement of striatal neuron activity in response to drugs of abuse.
Abstract: Synaptic plasticity in the striatum adjusts behaviour adaptively during skill learning, or maladaptively in the case of addiction. Just as dopamine plays a critical role in synaptic plasticity underlying normal skill learning and addiction, endogenous and exogenous opiates also modulate learning and addiction-related striatal plasticity. Though the role of opioid receptors in long-term depression in striatum has been characterized, their effect on long-term potentiation (LTP) remains unknown. In particular, direct pathway (dopamine D1 receptor-containing; D1R-) spiny projection neurons (SPNs) co-release the opioid neuropeptide dynorphin, which acts at presynaptic κ-opioid receptors (KORs) on dopaminergic afferents and can negatively regulate dopamine release. Therefore, we evaluated the interaction of co-released dynorphin and KOR on striatal LTP. We optogenetically facilitate the release of endogenous dynorphin from D1R-SPNs in brain slice while using whole-cell patch recording to measure changes in the synaptic response of SPNs following theta-burst stimulation (TBS) of cortical afferents. Our results demonstrate that TBS evokes corticostriatal LTP, and that optogenetic activation of D1R-SPNs during induction impairs LTP. Additional experiments demonstrate that optogenetic activation of D1R-SPNs reduces stimulation-evoked dopamine release and that bath application of a KOR antagonist provides full rescue of both LTP induction and dopamine release during optogenetic activation of D1R-SPNs. These results suggest that an increase in the opioid neuropeptide dynorphin is responsible for reduced TBS LTP and illustrate a physiological phenomenon whereby heightened D1R-SPN activity can regulate corticostriatal plasticity. Our findings have important implications for learning in addictive states marked by elevated direct pathway activation.
Keywords: dopamine; dynorphin; electrophysiology; striatum; synaptic plasticity; voltammetry.
Published 2017. This article is a U.S. Government work and is in the public domain in the USA.
Figures
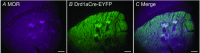
Comment in
-
'Synaptic autocontrol'.J Physiol. 2017 Aug 15;595(16):5405. doi: 10.1113/JP274545. Epub 2017 Jul 9. J Physiol. 2017. PMID: 28626995 Free PMC article. No abstract available.
Similar articles
-
Dichotomous regulation of striatal plasticity by dynorphin.Mol Psychiatry. 2023 Jan;28(1):434-447. doi: 10.1038/s41380-022-01885-0. Epub 2022 Dec 2. Mol Psychiatry. 2023. PMID: 36460726 Free PMC article.
-
Distinct roles of D1 and D5 dopamine receptors in motor activity and striatal synaptic plasticity.J Neurosci. 2003 Sep 17;23(24):8506-12. doi: 10.1523/JNEUROSCI.23-24-08506.2003. J Neurosci. 2003. PMID: 13679419 Free PMC article.
-
Dopaminergic control of synaptic plasticity in the dorsal striatum.Eur J Neurosci. 2001 Mar;13(6):1071-7. doi: 10.1046/j.0953-816x.2001.01485.x. Eur J Neurosci. 2001. PMID: 11285003 Review.
-
Coincidence of cholinergic pauses, dopaminergic activation and depolarisation of spiny projection neurons drives synaptic plasticity in the striatum.Nat Commun. 2022 Mar 11;13(1):1296. doi: 10.1038/s41467-022-28950-0. Nat Commun. 2022. PMID: 35277506 Free PMC article.
-
Dopamine-mediated regulation of corticostriatal synaptic plasticity.Trends Neurosci. 2007 May;30(5):211-9. doi: 10.1016/j.tins.2007.03.001. Epub 2007 Mar 23. Trends Neurosci. 2007. PMID: 17367873 Review.
Cited by
-
Long-Term Shaping of Corticostriatal Synaptic Activity by Acute Fasting.Int J Mol Sci. 2021 Feb 15;22(4):1916. doi: 10.3390/ijms22041916. Int J Mol Sci. 2021. PMID: 33671915 Free PMC article.
-
'Synaptic autocontrol'.J Physiol. 2017 Aug 15;595(16):5405. doi: 10.1113/JP274545. Epub 2017 Jul 9. J Physiol. 2017. PMID: 28626995 Free PMC article. No abstract available.
-
Opioidergic Modulation of Striatal Circuits, Implications in Parkinson's Disease and Levodopa Induced Dyskinesia.Front Neurol. 2018 Jul 5;9:524. doi: 10.3389/fneur.2018.00524. eCollection 2018. Front Neurol. 2018. PMID: 30026724 Free PMC article. Review.
-
Opioid Receptor-Mediated Regulation of Neurotransmission in the Brain.Front Mol Neurosci. 2022 Jun 15;15:919773. doi: 10.3389/fnmol.2022.919773. eCollection 2022. Front Mol Neurosci. 2022. PMID: 35782382 Free PMC article. Review.
-
Relevance of the Kappa Dynorphin System to Schizophrenia and Its Therapeutics.J Psychiatr Brain Sci. 2021;6:e210015. doi: 10.20900/jpbs.20210015. Epub 2021 Aug 24. J Psychiatr Brain Sci. 2021. PMID: 34532594 Free PMC article.
References
-
- Bagetta V, Picconi B, Marinucci S, Sgobio C, Pendolino V, Ghiglieri V, Fusco FR, Giampa C & Calabresi P (2011). Dopamine‐dependent long‐term depression is expressed in striatal spiny neurons of both direct and indirect pathways: implications for Parkinson's disease. J Neurosci 31, 12513–12522. - PMC - PubMed
-
- Barnes TD, Kubota Y, Hu D, Jin DZ & Graybiel AM (2005). Activity of striatal neurons reflects dynamic encoding and recoding of procedural memories. Nature 437, 1158–1161. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

