The mechanisms of radioresistance in esophageal squamous cell carcinoma and current strategies in radiosensitivity
- PMID: 28449496
- PMCID: PMC5394057
- DOI: 10.21037/jtd.2017.03.23
The mechanisms of radioresistance in esophageal squamous cell carcinoma and current strategies in radiosensitivity
Abstract
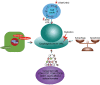
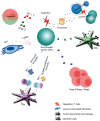
Esophageal cancer is the eighth most common cancer and the sixth leading cause of cancer-related death worldwide. Surgery is the primary form of treatment, but the survival is poor, especially for patients with locally advanced esophageal cancer. Radiotherapy has been a critical treatment option that may be combined with chemotherapy in patients with unresectable esophageal cancer. However, resistance to chemoradiotherapy might result in treatment failures and cancer relapse. This review will mainly focus on the possible cellular mechanisms and tumor-associated microenvironmental (TAM) factors that result in radioresistance in patients with esophageal cancer. In addition, current strategies to increase radiosensitivity, including targeted therapy and the use of radiosensitive biomarkers in clinical treatment, are discussed in this review.
Keywords: Esophageal cancer; radioresistance; radiosensitive biomarker; targeted therapy; tumor-associated microenvironment (TAM).
Conflict of interest statement
Conflicts of Interest: The authors have no conflicts of interest to declare.
Figures
References
-
- Ajani JA, D'Amico TA, Almhanna K, et al. Esophageal and esophagogastric junction cancers, version 1.2015. J Natl Compr Canc Netw 2015;13:194-227. - PubMed
-
- Miyata H, Doki Y, Shiozaki H, et al. CDC25B and p53 are independently implicated in radiation sensitivity for human esophageal cancers. Clin Cancer Res 2000;6:4859-65. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
