24-month clinical outcomes of a treat-and-extend regimen with ranibizumab for wet age-related macular degeneration in a real life setting
- PMID: 28449645
- PMCID: PMC5408417
- DOI: 10.1186/s12886-017-0451-1
24-month clinical outcomes of a treat-and-extend regimen with ranibizumab for wet age-related macular degeneration in a real life setting
Abstract
Background: To evaluate the clinical effectiveness and analyze the outcomes of a treat-and-extend (T&E) treatment regimen with ranibizumab for wet age-related macular degeneration (ARMD) in real life clinical settings over the first 2 years (24 months) of treatment.
Methods: Retrospective analysis of visual acuity, spectral domain optical coherence tomography (SD-OCT) parameters and treatment burden data of 56 eyes of 54 unselected treatment naive patients diagnosed with exudative ARMD. Monthly injections were offered until no signs of disease activity such as intra-retinal (IRF) or sub-retinal fluid (SRF) were evident on SD-OCT, followed by a gradual extension of the treatment interval by 2 weeks until a maximum of 12 weeks.
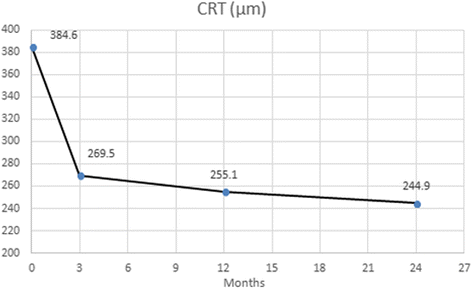
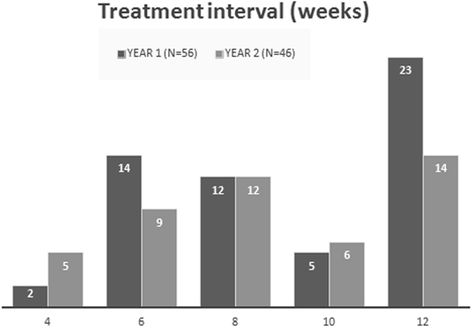
Results: The study met its main objective, demonstrating a mean best-corrected visual acuity gain of 8.3 letters (mean 68.8 ± 11) at month 12 and 5.2 letters (mean 65.7 ± 12.3) at 24 months compared to baseline (mean 60.5 ± 8.9). Anatomical improvement was also documented with a mean reduction of central retinal thickness by 139.7 μm at 24 months (244.9 ± 48.3) compared to baseline (384.6 ± 154.9). Forty-seven eyes (83.9% N = 56) gained vision or preserved baseline vision with 23 eyes (41.1%) gaining 10 letters or more at month 12. Out of the 46 eyes that completed 24 months of treatment and monitoring, 27 (58.7% N = 46) kept a BCVA above baseline with 18 of those (39% N = 46) maintaining a 10-letter gain throughout the 24 months. Six eyes (13% N = 46) lost more than 10 letters by month 24. The mean number of injections was 12.1 ± 2.8 over the 24-month period. Twenty-seven eyes (55.1% N = 56) achieved a treatment interval of 10 weeks or more at month 12, while the respective number at month 24 was 20 eyes (43.4% N = 46) in addition though to four more patients (8.7% N = 46) who were not receiving injections at month 24 since they were placed on a Monitor & Extend regime.
Conclusions: This is the first UK real-life study of a T&E treatment protocol with ranibizumab for exudative ARMD in a 24-month period and suggests that such a regimen is clinically effective and can achieve favourable outcomes with a significant reduction of the treatment burden compared to monthly PRN.
Keywords: Age related macular degeneration; Ranibizumab; Treat and extend.
Figures


References
-
- Evans JR, Fletcher AE, Wormald RP. Age-related macular degeneration causing visual impairment in people 75 years or older in Britain: an add-on study to the Medical Research Council Trial of Assessment and Management of Older People in the Community. Ophthalmology. 2004;111:513–517. doi: 10.1016/j.ophtha.2003.07.012. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

