YY-1224, a terpene trilactone-strengthened Ginkgo biloba, attenuates neurodegenerative changes induced by β-amyloid (1-42) or double transgenic overexpression of APP and PS1 via inhibition of cyclooxygenase-2
- PMID: 28449688
- PMCID: PMC5408406
- DOI: 10.1186/s12974-017-0866-x
YY-1224, a terpene trilactone-strengthened Ginkgo biloba, attenuates neurodegenerative changes induced by β-amyloid (1-42) or double transgenic overexpression of APP and PS1 via inhibition of cyclooxygenase-2
Abstract
Background: Ginkgo biloba has been reported to possess free radical-scavenging antioxidant activity and anti-inflammatory properties. In our pilot study, YY-1224, a terpene trilactone-strengthened extract of G. biloba, showed anti-inflammatory, neurotrophic, and antioxidant effects.
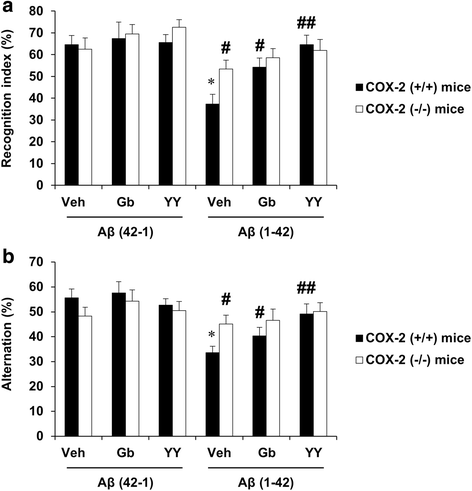
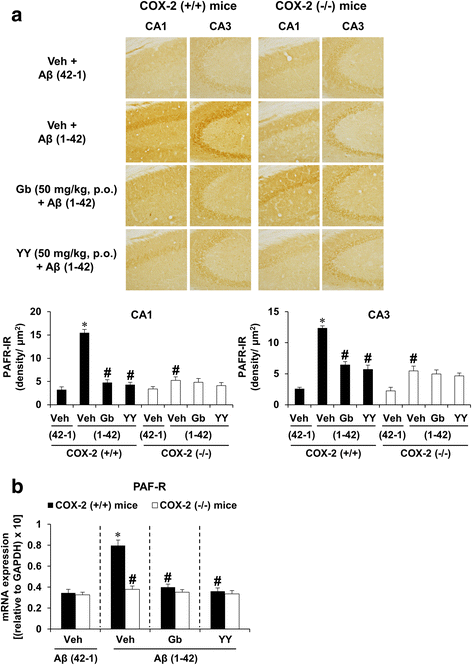
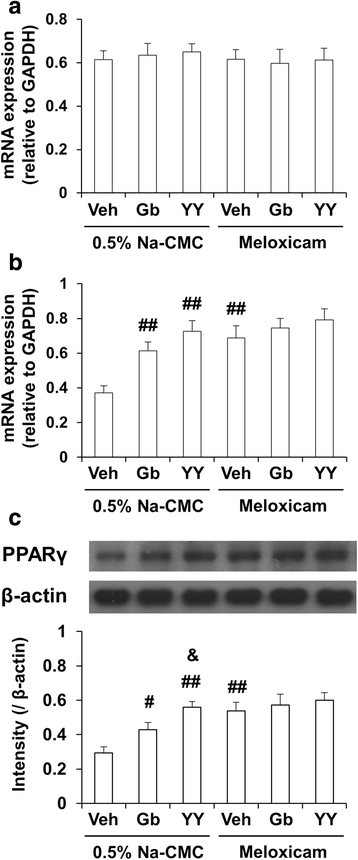
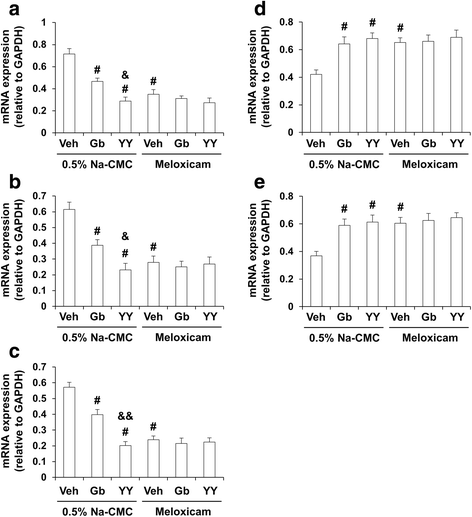
Results: We investigated the pharmacological potential of YY-1224 in β-amyloid (Aβ) (1-42)-induced memory impairment using cyclooxygenase-2 (COX-2) knockout (-/-) and APPswe/PS1dE9 transgenic (APP/PS1 Tg) mice. Repeated treatment with YY-1224 significantly attenuated Aβ (1-42)-induced memory impairment in COX-2 (+/+) mice, but not in COX-2 (-/-) mice. YY-1224 significantly attenuated Aβ (1-42)-induced upregulation of platelet-activating factor (PAF) receptor gene expression, reactive oxygen species, and pro-inflammatory factors. In addition, YY-1224 significantly inhibited Aβ (1-42)-induced downregulation of PAF-acetylhydrolase-1 (PAF-AH-1) and peroxisome proliferator-activated receptor γ (PPARγ) gene expression. These changes were more pronounced in COX-2 (+/+) mice than in COX-2 (-/-) mice. YY-1224 significantly attenuated learning impairment, Aβ deposition, and pro-inflammatory microglial activation in APP/PS1 Tg mice, whereas it significantly enhanced PAF-AH and PPARγ expression. A preferential COX-2 inhibitor, meloxicam, did not affect the pharmacological activity by YY-1224, suggesting that the COX-2 gene is a critical mediator of the neuroprotective effects of YY-1224. The protective activity of YY-1224 appeared to be more efficacious than a standard G. biloba extract (Gb) against Aβ insult.
Conclusions: Our results suggest that the protective effects of YY-1224 against Aβ toxicity may be associated with its PAF antagonistic- and PPARγ agonistic-potential as well as inhibition of the Aβ-mediated pro-inflammatory switch of microglia phenotypes through suppression of COX-2 expression.
Keywords: APPswe/PS1dE9 transgenic mice; Cyclooxygenase-2 knockout mice; Microglia; Peroxisome proliferators-activated receptor γ; Platelet-activating factor; Terpene trilactone-strengthened G. biloba.
Figures









Similar articles
-
Neuroprotective effects of verbascoside against Alzheimer's disease via the relief of endoplasmic reticulum stress in Aβ-exposed U251 cells and APP/PS1 mice.J Neuroinflammation. 2020 Oct 18;17(1):309. doi: 10.1186/s12974-020-01976-1. J Neuroinflammation. 2020. PMID: 33070776 Free PMC article.
-
Long-term treatment with Ginkgo biloba extract EGb 761 improves symptoms and pathology in a transgenic mouse model of Alzheimer's disease.Brain Behav Immun. 2015 May;46:121-31. doi: 10.1016/j.bbi.2015.01.011. Epub 2015 Jan 28. Brain Behav Immun. 2015. PMID: 25637484
-
EGb761 improves cognitive function and regulates inflammatory responses in the APP/PS1 mouse.Exp Gerontol. 2016 Aug;81:92-100. doi: 10.1016/j.exger.2016.05.007. Epub 2016 May 22. Exp Gerontol. 2016. PMID: 27220811
-
Ginkgo biloba and Its Chemical Components in the Management of Alzheimer's Disease.Am J Chin Med. 2024;52(3):625-666. doi: 10.1142/S0192415X24500277. Epub 2024 Apr 24. Am J Chin Med. 2024. PMID: 38654507 Review.
-
[Ginkgolide B: mechanisms of neurobiological effects, prospects for use in the therapy of Alzheimer's disease].Zh Nevrol Psikhiatr Im S S Korsakova. 2024;124(4):22-27. doi: 10.17116/jnevro202412404122. Zh Nevrol Psikhiatr Im S S Korsakova. 2024. PMID: 38676673 Review. Russian.
Cited by
-
New Insights Into the Pathologic Roles of the Platelet-Activating Factor System.Front Endocrinol (Lausanne). 2021 Mar 15;12:624132. doi: 10.3389/fendo.2021.624132. eCollection 2021. Front Endocrinol (Lausanne). 2021. PMID: 33796070 Free PMC article. Review.
-
Anti-Aging of the Nervous System and Related Neurodegenerative Diseases With Chinese Herbal Medicine.Am J Alzheimers Dis Other Demen. 2023 Jan-Dec;38:15333175231205445. doi: 10.1177/15333175231205445. Am J Alzheimers Dis Other Demen. 2023. PMID: 37818604 Free PMC article. Review.
-
The Multifaceted Role of Neuroprotective Plants in Alzheimer's Disease Treatment.Geriatrics (Basel). 2022 Feb 26;7(2):24. doi: 10.3390/geriatrics7020024. Geriatrics (Basel). 2022. PMID: 35314596 Free PMC article. Review.
-
Insight into Ginkgo biloba L. Extract on the Improved Spatial Learning and Memory by Chemogenomics Knowledgebase, Molecular Docking, Molecular Dynamics Simulation, and Bioassay Validations.ACS Omega. 2020 Jan 28;5(5):2428-2439. doi: 10.1021/acsomega.9b03960. eCollection 2020 Feb 11. ACS Omega. 2020. PMID: 32064403 Free PMC article.
-
Phenolic composition and neuroprotective effects of the ethyl-acetate fraction from Inonotus sanghuang against H2O2-induced apoptotic cell death of primary cortical neuronal cells.Food Sci Biotechnol. 2022 Jun 24;31(9):1213-1223. doi: 10.1007/s10068-022-01107-x. eCollection 2022 Aug. Food Sci Biotechnol. 2022. PMID: 35919355 Free PMC article.
References
-
- Mouri A, Noda Y, Hara H, Mizoguchi H, Tabira T, Nabeshima T. Oral vaccination with a viral vector containing Abeta cDNA attenuates age-related Abeta accumulation and memory deficits without causing inflammation in a mouse Alzheimer model. FASEB J. 2007;21:2135–48. doi: 10.1096/fj.06-7685com. - DOI - PubMed
-
- Akiyama H, Barger S, Barnum S, Bradt B, Bauer J, Cole GM, Cooper NR, Eikelenboom P, Emmerling M, Fiebich BL, Finch CE, Frautschy S, Griffin WS, Hampel H, Hull M, Landreth G, Lue L, Mrak R, Mackenzie IR, McGeer PL, O'Banion MK, Pachter J, Pasinetti G, Plata-Salaman C, Rogers J, Rydel R, Shen Y, Streit W, Strohmeyer R, Tooyoma I, Van Muiswinkel FL, Veerhuis R, Walker D, Webster S, Wegrzyniak B, Wenk G, Wyss-Coray T. Inflammation and Alzheimer’s disease. Neurobiol Aging. 2000;21:383–421. doi: 10.1016/S0197-4580(00)00124-X. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

