Altered Ca2+ homeostasis induces Calpain-Cathepsin axis activation in sporadic Creutzfeldt-Jakob disease
- PMID: 28449707
- PMCID: PMC5408381
- DOI: 10.1186/s40478-017-0431-y
Altered Ca2+ homeostasis induces Calpain-Cathepsin axis activation in sporadic Creutzfeldt-Jakob disease
Abstract
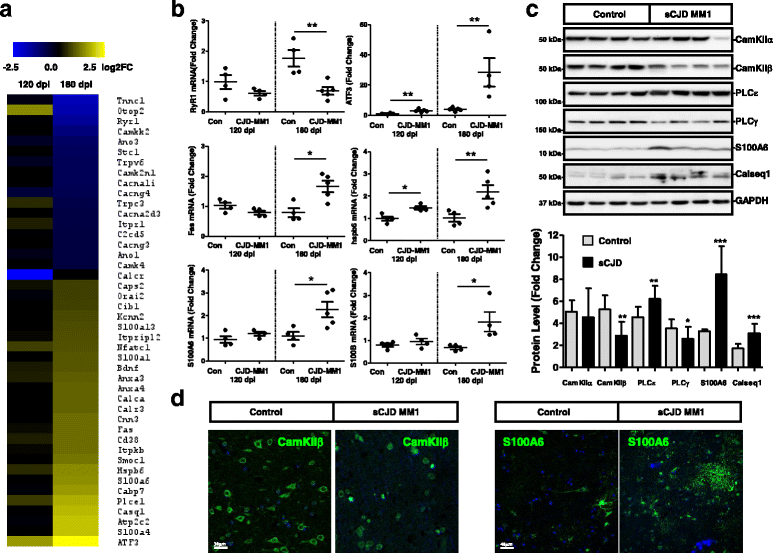
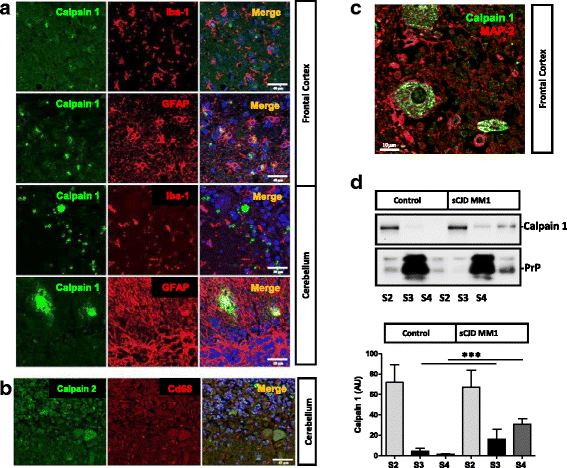
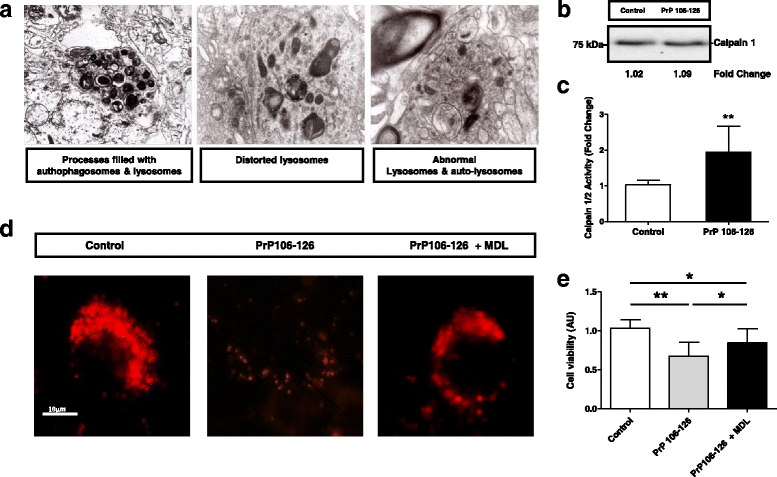
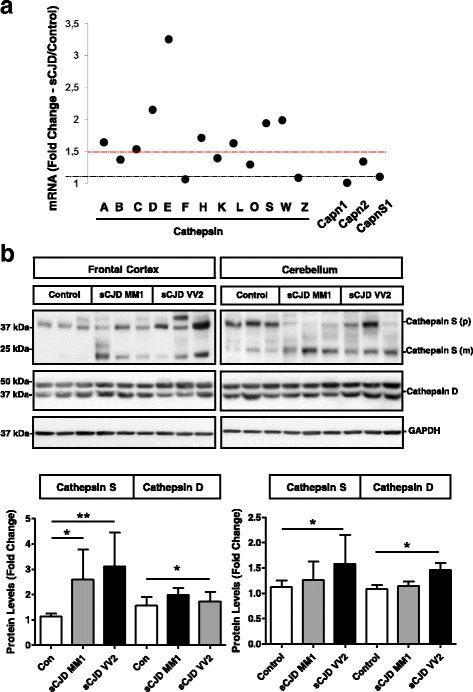
Sporadic Creutzfeldt-Jakob disease (sCJD) is the most prevalent form of human prion disease and it is characterized by the presence of neuronal loss, spongiform degeneration, chronic inflammation and the accumulation of misfolded and pathogenic prion protein (PrPSc). The molecular mechanisms underlying these alterations are largely unknown, but the presence of intracellular neuronal calcium (Ca2+) overload, a general feature in models of prion diseases, is suggested to play a key role in prion pathogenesis.Here we describe the presence of massive regulation of Ca2+ responsive genes in sCJD brain tissue, accompanied by two Ca2+-dependent processes: endoplasmic reticulum stress and the activation of the cysteine proteases Calpains 1/2. Pathogenic Calpain proteins activation in sCJD is linked to the cleavage of their cellular substrates, impaired autophagy and lysosomal damage, which is partially reversed by Calpain inhibition in a cellular prion model. Additionally, Calpain 1 treatment enhances seeding activity of PrPSc in a prion conversion assay. Neuronal lysosomal impairment caused by Calpain over activation leads to the release of the lysosomal protease Cathepsin S that in sCJD mainly localises in axons, although massive Cathepsin S overexpression is detected in microglial cells. Alterations in Ca2+ homeostasis and activation of Calpain-Cathepsin axis already occur at pre-clinical stages of the disease as detected in a humanized sCJD mouse model.Altogether our work indicates that unbalanced Calpain-Cathepsin activation is a relevant contributor to the pathogenesis of sCJD at multiple molecular levels and a potential target for therapeutic intervention.
Keywords: Ca2+; Calcium; Calpain; Cathepsin; Creutzfeldt-Jakob disease; Prion protein.
Figures


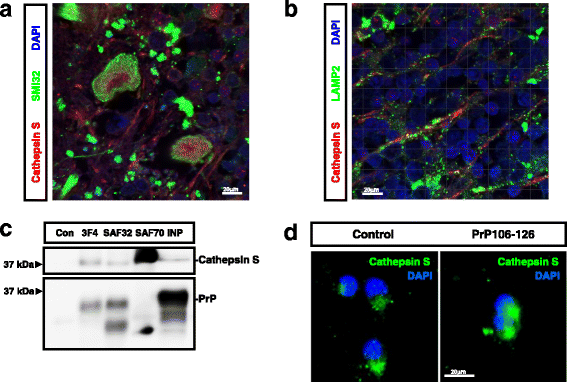

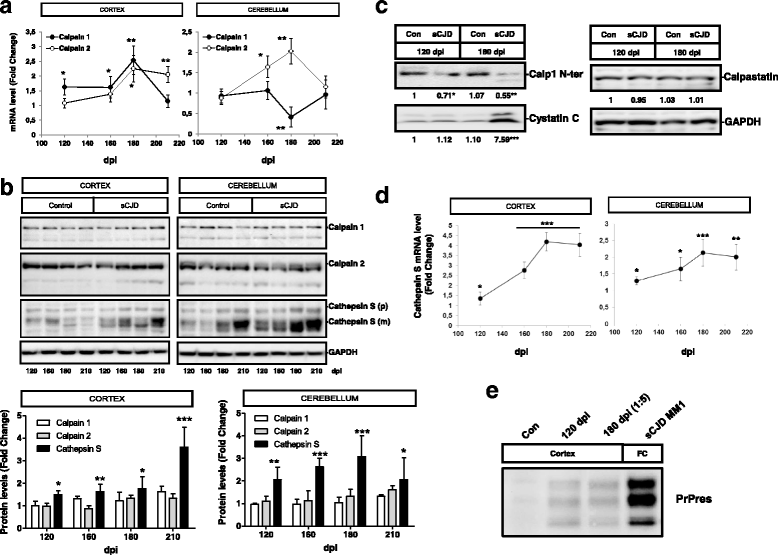
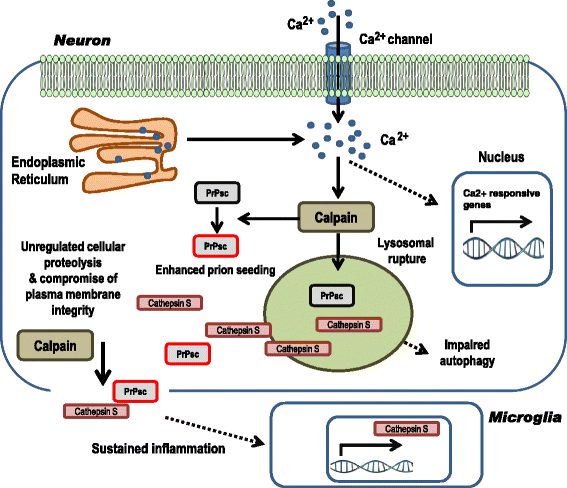
References
-
- Ansoleaga B, Garcia-Esparcia P, Llorens F, Hernandez-Ortega K, Carmona M, Del Rio JA, Zerr I, Ferrer I. Altered mitochondria, protein synthesis machinery, and Purine metabolism are molecular contributors to the pathogenesis of Creutzfeldt-Jakob disease. J Neuropathol Exp Neurol. 2016;75:755–769. doi: 10.1093/jnen/nlw048. - DOI - PubMed
-
- Atarashi R, Satoh K, Sano K, Fuse T, Yamaguchi N, Ishibashi D, Matsubara T, Nakagaki T, Yamanaka H, Shirabe S, Yamada M, Mizusawa H, Kitamoto T, Klug G, McGlade A, Collins SJ, Nishida N. Ultrasensitive human prion detection in cerebrospinal fluid by real-time quaking-induced conversion. Nat Med. 2011;17:175–8. doi: 10.1038/nm.2294. - DOI - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

