Loss of WDFY3 ameliorates severity of serum transfer-induced arthritis independently of autophagy
- PMID: 28449847
- PMCID: PMC5515728
- DOI: 10.1016/j.cellimm.2017.04.001
Loss of WDFY3 ameliorates severity of serum transfer-induced arthritis independently of autophagy
Abstract
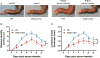
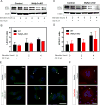
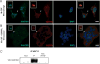
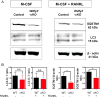
WDFY3 is a master regulator of selective autophagy that we recently showed to interact with TRAF6 and augment RANKL-induced osteoclastogenesis in vitro and in vivo via the NF-κB pathway. Since the NF-κB pathway plays a major role in inflammation herein, we investigate the role of WDFY3 in an arthritis animal model. Our data show that WDFY3 conditional knockout mice (Wdfy3loxP/loxP-LysM-Cre+) were protected in the K/BxN serum transfer-induced arthritis animal model. These effects were independent of alterations in starvation-induced autophagy as evidenced by Western blot analysis of the autophagy marker LC3, autophagosome formation in osteoclast precursors and lysosome formation in osteoclasts derived from WDFY3-cKO mice compared to controls. Moreover, we demonstrate by immunofluorescence and co-immunoprecipitation that WDFY3 interacts with SQSTM1 in macrophages and osteoclasts. Collectively, our data suggest that loss of WDFY3 in myeloid cells leads to reduced severity of inflammatory arthritis independently of WDFY3 function in starvation-induced autophagy.
Keywords: ALFY; Autophagy; Autophagy-linked FYVE containing protein; Musculoskeletal diseases; Osteoclast; WDFY3.
Copyright © 2017 Elsevier Inc. All rights reserved.
Conflict of interest statement
None of the authors has any potential financial conflict of interest related to this manuscript.
Figures
References
-
- Gutierrez MG, Master SS, Singh SB, Taylor GA, Colombo MI, Deretic V. Autophagy is a defense mechanism inhibiting BCG and Mycobacterium tuberculosis survival in infected macrophages. Cell. 2004;119:753–766. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

