Migration pathways of sacral neural crest during development of lower urogenital tract innervation
- PMID: 28449850
- PMCID: PMC5572097
- DOI: 10.1016/j.ydbio.2017.04.011
Migration pathways of sacral neural crest during development of lower urogenital tract innervation
Abstract
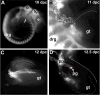
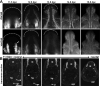
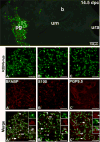
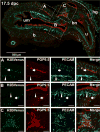
The migration and fate of cranial and vagal neural crest-derived progenitor cells (NCPCs) have been extensively studied; however, much less is known about sacral NCPCs particularly in regard to their distribution in the urogenital system. To construct a spatiotemporal map of NCPC migration pathways into the developing lower urinary tract, we utilized the Sox10-H2BVenus transgene to visualize NCPCs expressing Sox10. Our aim was to define the relationship of Sox10-expressing NCPCs relative to bladder innervation, smooth muscle differentiation, and vascularization through fetal development into adulthood. Sacral NCPC migration is a highly regimented, specifically timed process, with several potential regulatory mileposts. Neuronal differentiation occurs concomitantly with sacral NCPC migration, and neuronal cell bodies are present even before the pelvic ganglia coalesce. Sacral NCPCs reside within the pelvic ganglia anlagen through 13.5 days post coitum (dpc), after which they begin streaming into the bladder body in progressive waves. Smooth muscle differentiation and vascularization of the bladder initiate prior to innervation and appear to be independent processes. In adult bladder, the majority of Sox10+ cells express the glial marker S100β, consistent with Sox10 being a glial marker in other tissues. However, rare Sox10+ NCPCs are seen in close proximity to blood vessels and not all are S100β+, suggesting either glial heterogeneity or a potential nonglial role for Sox10+ cells along vasculature. Taken together, the developmental atlas of Sox10+ NCPC migration and distribution profile of these cells in adult bladder provided here will serve as a roadmap for future investigation in mouse models of lower urinary tract dysfunction.
Keywords: Autonomic nervous system; Bladder; Lower urinary tract; Pelvic ganglia; Peripheral nervous system; Sacral neural crest; Sox10.
Copyright © 2017 Elsevier Inc. All rights reserved.
Figures





Similar articles
-
Altered sacral neural crest development in Pax3 spina bifida mutants underlies deficits of bladder innervation and function.Dev Biol. 2021 Aug;476:173-188. doi: 10.1016/j.ydbio.2021.03.024. Epub 2021 Apr 9. Dev Biol. 2021. PMID: 33839113 Free PMC article.
-
Direct Interaction of Sox10 With Cadherin-19 Mediates Early Sacral Neural Crest Cell Migration: Implications for Enteric Nervous System Development Defects.Gastroenterology. 2022 Jan;162(1):179-192.e11. doi: 10.1053/j.gastro.2021.08.029. Epub 2021 Aug 21. Gastroenterology. 2022. PMID: 34425092
-
Phenotypes of neural-crest-derived cells in vagal and sacral pathways.Cell Tissue Res. 2006 Jan;323(1):11-25. doi: 10.1007/s00441-005-0047-6. Epub 2005 Aug 30. Cell Tissue Res. 2006. PMID: 16133146
-
Signaling pathways bridging fate determination of neural crest cells to glial lineages in the developing peripheral nervous system.Cell Signal. 2014 Apr;26(4):673-82. doi: 10.1016/j.cellsig.2013.12.007. Epub 2013 Dec 27. Cell Signal. 2014. PMID: 24378534 Review.
-
The importance of having your SOX on: role of SOX10 in the development of neural crest-derived melanocytes and glia.Oncogene. 2003 May 19;22(20):3024-34. doi: 10.1038/sj.onc.1206442. Oncogene. 2003. PMID: 12789277 Review.
Cited by
-
An extended regulatory landscape drives Tbx18 activity in a variety of prostate-associated cell lineages.Dev Biol. 2019 Feb 15;446(2):180-192. doi: 10.1016/j.ydbio.2018.11.023. Epub 2018 Dec 27. Dev Biol. 2019. PMID: 30594504 Free PMC article.
-
Neural crest-derived neurons invade the ovary but not the testis during mouse gonad development.Proc Natl Acad Sci U S A. 2019 Mar 19;116(12):5570-5575. doi: 10.1073/pnas.1814930116. Epub 2019 Feb 28. Proc Natl Acad Sci U S A. 2019. PMID: 30819894 Free PMC article.
-
Lrig2 and Hpse2, mutated in urofacial syndrome, pattern nerves in the urinary bladder.Kidney Int. 2019 May;95(5):1138-1152. doi: 10.1016/j.kint.2018.11.040. Epub 2019 Mar 8. Kidney Int. 2019. PMID: 30885509 Free PMC article.
-
hPSC-derived sacral neural crest enables rescue in a severe model of Hirschsprung's disease.Cell Stem Cell. 2023 Mar 2;30(3):264-282.e9. doi: 10.1016/j.stem.2023.02.003. Cell Stem Cell. 2023. PMID: 36868194 Free PMC article.
-
Innervation in organogenesis.Curr Top Dev Biol. 2022;148:195-235. doi: 10.1016/bs.ctdb.2022.02.004. Epub 2022 Mar 12. Curr Top Dev Biol. 2022. PMID: 35461566 Free PMC article.
References
-
- Anderson RB, Stewart AL, Young HM. Phenotypes of neural-crest-derived cells in vagal and sacral pathways. Cell Tissue Res. 2006;323:11–25. - PubMed
-
- Atala A. Tissue engineering of human bladder. Br Med Bull. 2011;97:81–104. - PubMed
-
- Atala A, Bauer SB, Soker S, Yoo JJ, Retik AB. Tissue-engineered autologous bladders for patients needing cystoplasty. Lancet. 2006;367:1241–1246. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

